It’s that time of year again when we bring you our Annual Report on the contact lens industry, with a particular focus on 2016. As you will see, we’ve observed some similar trends to what we have reported in years past—but you may come across some unexpected findings as well. A lot happened in 2016, so dig in and get ready to build up your contact lens practice with these findings.
OVERVIEW OF GENERAL MARKET TRENDS
The size of the contact lens market is always tough to pin down. Data obtained from Robert W. Baird (Jeff Johnson, OD, CFA, managing director, senior research analyst) shows that as of the third quarter of 2016, contact lens trends have been healthy on a marketwide basis, with U.S. sales growing nearly 5% through the first nine months of the year and worldwide sales growing a similar 4% to 5% (after excluding the impact of recent fluctuations in foreign exchange rates).
As for the contact lens market size, Baird’s 2016 data suggest that the value of the worldwide contact lens market currently stands at roughly $7.2 billion, with the U.S. market valued at slightly more than $2.5 billion.
CURRENT PRACTICE TRENDS
Each year, Contact Lens Spectrum conducts its own market research through a Reader Profile survey. In the survey, we ask our readers about their practice trends and patterns both generally and as they relate specifically to contact lens practice. Because we have the benefit of several years of past responses, we are also able to conduct some longer-term and longitudinal analyses. Survey questions ask about characteristics of the patient base of a practice, business and financial aspects of a practice, fitting and prescribing trends, and care solution trends, among other topics. This year, we had close to 150 U.S. respondents who accessed the survey link. Going forward, I will use our research, as well as data from other sources, to pinpoint trends and discuss observations about the contact lens field.
Practice and Business Trends Table 1 summarizes trends in the practice and business characteristics from 2009 to 2016. Optometrists topped our list of respondents (81%), followed by opticians, contact lens technicians, and ophthalmologists, respectively. While modes of practice varied, the most common was solo private practice (43%), followed by group private practice (27%), and independent affiliation with a retail setting (6%). In 2016, the typical practice saw an average of 105 patients per week, which is marginally down from last year. Additionally, the average number of contact lens fittings and refitting in a typical week remained almost constant at 26.
| 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | |
|---|---|---|---|---|---|---|---|---|
| Patients Seen Each Week | 108 | 116 | 107 | 127 | 125 | 117 | 124 | 105 |
| % Contact Lens-Wearing Patients | 37 | 36 | 35 | 34 | 34 | 34 | 49 | 33 |
| # CL Fits/Refits Per Week | 27 | 27 | 24 | 26 | 25 | 24 | 29 | 26 |
| Estimated % Gross Practice Revenue from CLs | 35 | 34 | 37 | 32 | 30 | 30 | 39 | 32 |
| Estimated % Net Practice Revenue from CLs | 29 | 28 | 26 | 27 | 25 | 25 | 27 | 27 |
Just like last year, most of 2016’s respondents felt that about 32% of their gross profit and about 27% of their net profit was derived from the contact lens portion of their practices. New data this year (Figure 1) found that practitioners estimate that 67% of their patients purchase contact lenses from their practice, whereas 18% purchase their contact lenses online, 13% purchase their lenses through a third-party retailer independent of a practice, and 2% purchase their contact lenses from another practice setting.

In addition, 63% of practitioners believe that they will see an increase in their overall contact lens practice in 2017, while only 37% believe it will stay the same; no one indicated that it will be decreasing further.
Lens Dispensing and Mode of Wear Trends Just as in years past, our data show that silicone hydrogel materials make up the majority of the fits and refits that are conducted today (Figure 2). About five years ago, we noticed a slowing of the silicone hydrogel category; for 2016, however, we note that silicone hydrogels are being reported in usage at 67% (nearly identical to the last few years) while hydrogels were reportedly used in 20% of fits. Up slightly from 2015, data from Contact Lens Spectrum’s market analysis showed that 10% of fits and refits were conducted with a GP; note that respondents previously indicated that only 6% of fits and refits were done with GPs in 2014.

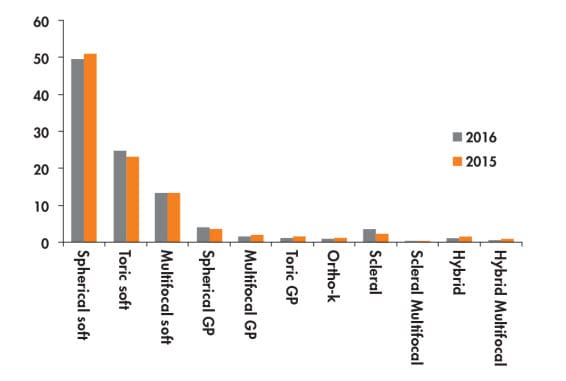
Data from Contact Lens Spectrum’s market research show that, across all contact lens designs, most of the reported fits and refits are with soft spherical lenses (49% versus 51% in 2015), followed by soft toric lenses (25% versus 23% in 2015), soft multifocal lenses (13%), spherical corneal GPs (4%), and scleral designs (4%) (Figure 3). This is the first year that we observed scleral lenses being fitted with the same frequency as spherical corneal GP designs and with greater frequency than both toric and multifocal GP designs combined.
Along these same lines, when we asked about the greatest growth potential of several popular specialty lens options in 2017, most practitioners indicated scleral lenses (40%, compared to 37% for 2015), followed by custom soft lenses (33%, which was the same as last year), followed by orthokeratology (ortho-k; 14%, which was almost equal to 2015), and hybrids (13%, which was a slight decline from last year’s 17%).
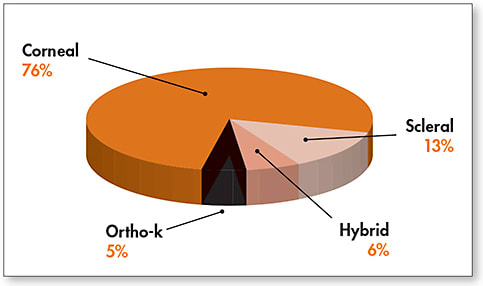
When we asked practitioners to estimate the distribution of lenses by category of lens design for contact lenses containing any GP lens material (Figure 4), it is not surprising that corneal designs made up the bulk of fits (76%), followed by sclerals (13%), hybrids (6%), and ortho-k (5%).
When comparing the four major soft lens categories (spherical, toric, multifocal, and cosmetic), similar trends can be seen in data obtained from ABB Optical Group (an independent optical industry platform), GfK Retail and Technology (a market research service), and Glimpse Live, LLC (a provider of performance dashboards and analytics). As noted in Table 2, data from these sources show slight variability when looking at the soft spherical category prescribing (range 53% to 62%) and for multifocal soft lens prescribing (range 8% to 18%); the data show a little more consistency in prescribing habits with soft torics (range 26% to 27%) and soft cosmetic lenses (range 3% to 4%). When Contact Lens Spectrum readers were asked which soft lens categories they anticipated fitting more of in 2017, 57% indicated daily disposables, followed by multifocals (38%), cosmetic (2.5%), and torics (2.5%).
| SOFT LENS CATEGORY | Contact Lens Spectrum | ABB Optical Group | GfK Retail & Technology | Glimpse Live | Change from 2015 | ||
|---|---|---|---|---|---|---|---|
| ABB | GfK | Glimpse | |||||
| Spherical | 53% | 59% | 62% | 60% | –2% | 0.4% | –3% |
| Toric | 26% | 27% | 27% | 27% | 1% | 4.2% | –1% |
| Multifocal | 18% | 11% | 8% | 10% | 1% | 0.2% | 3% |
| Cosmetic | 4% | 3% | 3% | 3% | –0.1% | –3.4% | –11% |
| Data from ABB, GfK, and Glimpse are percent of sales. | |||||||
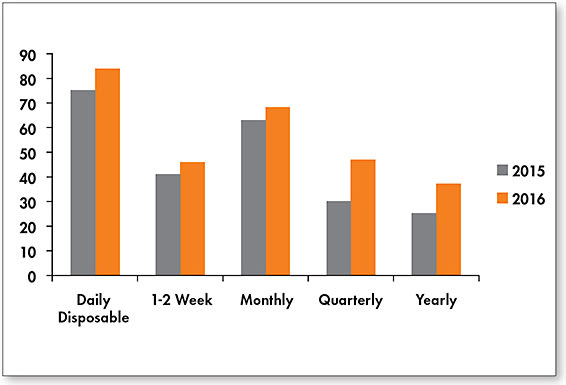
In addition to the Contact Lens Spectrum Reader Profile Survey (Figure 5), market insights were also gleaned from ABB Optical Group, GfK Retail and Technology, and Glimpse Live in terms of replacement schedule usage (Table 3). When comparing numbers among the sources, interesting trends emerge—for two of the four sources, the daily disposable modality emerged as the one leading in terms of prescribing by soft lens replacement schedule (range 31% to 39%) followed by the monthly category (range 28% to 44%). The weekly/two-week category continues to show declines (ranging from –2% to –12%), and the daily disposable category continues to be associated with the most growth (ranging from 5% to 20%)—a trend we have observed for the last few years.

| SOFT LENS CATEGORY | Contact Lens Spectrum | ABB Optical Group | GfK Retail & Technology | Glimpse Live | Change from 2015 | ||
|---|---|---|---|---|---|---|---|
| ABB | GfK | Glimpse | |||||
| Daily | 31% | 38% | 31% | 39% | 5% | 13.7% | 13% |
| Weekly/Two-Week | 24% | 25% | 31% | 33% | –3% | –6.2% | –12% |
| Monthly | 44% | 36% | 38% | 28% | –2% | –1% | 0% |
| Conventional | 1% | 1% | 0.2% | 0.2% | –0.1% | –13.9% | –14% |
| Data from ABB, GfK, and Glimpse are percent of sales. | |||||||
When we asked about the contact lens design or modality with the greatest growth potential/anticipated use over the next year, 83% of respondents in our market research indicated silicone hydrogel daily disposables (up a little from 78% in 2015), followed by silicone hydrogel multifocals (67%, which is close to last year’s 63%), and sclerals (41%). In addition, anticipated use of hydrogel daily disposables and silicone hydrogel torics seemed to flip flop from last year at 51% versus 42% and 43% versus 50%, respectively.
As has been the case for several years now, for presbyopic patients wearing contact lenses, most practitioners continue to strongly prefer multifocal lenses (76% in 2016, compared to 71% in 2015) compared with monovision (17% in 2016, compared to 19% in 2015) and over-spectacles (8% for 2016 and 10% for 2015). In practice, more of your presbyopic patients are prescribed a multifocal (44% of your contact lens-wearing presbyopes versus 48% in 2015) compared with monovision (25% of your contact lens-wearing presbyopes versus 31% in 2015).
Myopia control with contact lenses continues to grow among contact lens-prescribing eyecare practitioners. In 2016, 37% of Contact Lens Spectrum Reader Profile respondents indicated that they actively practice myopia control with contact lenses (up from only 24% in 2015). Of those who are practicing myopia control (Figure 6), soft multifocal designs come in on top (51%), followed by ortho-k (44%) and GP multifocals (5%). While ortho-k ranked the highest in 2015, this year’s results showing a preference for soft multifocals more closely mimic 2014.

Of the categories noted earlier, there were three types of contact lenses that the majority of respondents predict would be used more when considering the year ahead (compared to either staying the same or decreasing): silicone hydrogel daily disposables (83% of practitioners), silicone hydrogel multifocals (67% of practitioners), and hydrogel daily disposables (51%). The only category in which the majority of respondents felt that their prescribing would decrease is in the hydrogel one- and two-week category (54%). Interestingly, although practitioners anticipate using the silicone hydrogel materials and designs to a greater extent, it has not translated into measurable changes in the percentage that the material category is used as a whole (Figure 2); this is very similar to last year.
CONTACT LENS WEAR AND CARE COMPLIANCE
According to Contact Lens Spectrum’s research, practitioners indicated that only 46% of their patients using one- to two-week replacement lenses were compliant with the replacement schedule, whereas practitioners indicated that 68% of their patients using monthly lenses were compliant, and 84% of those using daily disposable lenses were compliant (Figure 7). When looking at the overall contact lens patient population, however, practitioners indicated that about 69% of those patients in their practice properly comply with replacement per instruction. The trends observed this year have not changed substantially from previous years.
LENS CARE TRENDS
Our survey also indicated that about three-quarters of respondents reported using chemical care systems with contact lens patients (73% in 2016, up only 1% over 2015), followed by hydrogen peroxide-based systems (27% in both 2015 and 2016). Not surprisingly, this is the same trend that we have seen going back to 2009 (Figure 8). Our data also show that practitioners are recommending care systems when prescribing contact lenses—88% recommend specific brands of contact lens care systems, and only 12% do not. This statistic represents a small 2% increase over last year for those who are recommending specific care systems. The largest factor influencing their recommendation is improved comfort (28%), followed by lens disinfection efficacy (25%), material/solution compatibility (23%), and then cleaning efficacy (12%), convenience (12%), and cost (1%).

YESTERDAY AND TODAY
To put all of these statistics in context, it’s always important to look at where the industry was previously. Following tradition, we will journey back and peruse the most newsworthy topics of a decade ago.
The 2006 Event of the Year was the global recall and discontinuation of manufacturing and sales of ReNu with MoistureLoc by Bausch & Lomb. When deciding on that event, then-editor Dr. Joseph Barr said “What had become the most successful launch of a new product in the history of contact lens solutions, and a solution that truly did make silicone hydrogel lenses more wettable clinically, ended with a spike in Fusarium infections and a legal nightmare for many. But that is past and we’ve all been sobered and reminded in no uncertain terms about the importance of safe, biocompatible, well-tested contact lens care products, and how they protect and help our patients succeed in contact lens wear.”
He also noted that some industry experts were concerned that sensationalized discussions and lens solution recalls in 2006 would bode unfavorably for a healthy contact lens industry in 2007. Instead, however, that was not the case. In fact, many practitioners still look to this event as a way to broach the topic of the importance of proper lens care with their patients.
Silicone hydrogel (SiHy) lenses were another buzzworthy topic in 2006. In his January 2006 editorial, Dr. Barr even questioned whether SiHy lenses would replace hydrogels in this decade—because, he noted, it looked like the statistics were trending in that direction. He wasn’t alone in that thought either. When asked “What modality do you see increasing?” in Contact Lens Spectrum’s 2006 Reader Profile Survey, 72% of respondents indicated SiHy lenses.
Spherical SiHy lens sales increased more than 50% in 2006, with an accompanying 10% decrease in hydrogel lens sales overall (Barr, 2007). Additionally, in 2006, SiHy lenses accounted for 37% of retail contact lens sales.
And, while the SiHy category has increased substantially every year through 2011, there has been some leveling off over the past five years (Nichols, 2016). As noted earlier, this year’s usage numbers for SiHy and hydrogel lenses comes in at 67% and 20%, respectively.
The third news item on everyone’s minds in 2006 was the implementation of the Fairness to Contact Lens Consumers Act (FCLCA) and the Contact Lens Rule. According to the Federal Trade Commission (FTC), the act gives consumers certain rights, imposes duties on contact lens prescribers and sellers, and requires the FTC to develop and enforce implementing rules (www.ftc.gov/tips-advice/business-center/guidance/contact-lens-rule-guide-prescribers-sellers ).
In 2006, the FTC began enforcing the act and filing charges against several companies for violations of the FCLCA. In April 2006, Sen. Robert Bennett (R-UT) and Sen. Patrick Leahy (D-VT) introduced S. 2480, The Contact Lens Consumer Protection Act (introduced in Congress as HR 5762), which sought to amend the FCLCA to include restrictions on manufacturer distribution policies, but this bill was opposed by many involved parties due to the passive verification included in the bill. Then, that September, Rep. Ed Whitfield (RKY) introduced HR 6117, the Contact Lens Consumer Health Protection Act, a bill to protect consumers who buy contact lenses from third-party vendors. The bill would amend the FCLCA and establish a toll-free Patient Safety Hotline for eyecare practitioners who have patient health concerns related to a prescription verification request.
Now, we are seeing this legislation brought up again as part of the FTC’s mandated 10-year regulatory review schedule. On Sept. 22, 2016, HR 6157 (introduced by Rep. Pete Olson [R-TX] and Rep. Kathy Castor [D-FL]) sought to modernize the prescription verification process for contact lenses and clarify consumer protections regarding their false advertising. This bill complements S 2777, the Contact Lens Consumer Health Protection Act of 2016, which was introduced in April by Sen. Bill Cassidy, MD, (R-LA) (www.aoa.org/news/advocacy/legislation-targets-prescription-verification-deceptive-internet-sales-tactics?sso=y ). HR 6157 would amend the FCLCA.
So far, as part of its review, the FTC proposes to amend the Contact Lens Rule to require that prescribers obtain a signed acknowledgment after releasing a contact lens prescription to a patient and maintain each such acknowledgment for a period of at least three years. The FTC seeks comment on this proposal and several other issues, such as additional mechanisms for improving prescription portability; additional copies of prescriptions; and sellers designated to act on behalf of patients. Comments can be submitted at ftcpublic.commentworks.com/ftc/contactlensrule until Jan. 30, 2017.
TOMORROW
Looking forward, there are several hot button topics that have made headlines this year and are sure to stick around through 2017 and beyond.
First, there were sparks of activity related to dry eye disease (DED) in 2016. Besides gaining street cred from actress Jennifer Aniston’s confession to being a dry eye sufferer, 2016 saw several new dry eye treatment offerings. Novaliq GmbH completed enrollment in its Phase 2 clinical trial that is evaluating the safety, efficacy, and tolerability of CyclASol for the treatment of moderate-to-severe DED, and its topline results were anticipated at the end of 2016. Auven Therapeutics announced results from its Phase 2b/3 clinical trial evaluating the safety and efficacy of Seciera (OTX-101), a novel, investigational nanomicellar formulation of cyclosporine utilizing patent-protected proprietary technology to enhance penetration into target tissues of the eye. If these studies are successful, the submission of a New Drug Application for Seciera for treatment of DED is anticipated in early 2017. And, the U.S. Food and Drug Administration (FDA) approved Shire plc’s Xiidra (lifitegrast ophthalmic solution) 5%, a twice-daily eye drop solution indicated for the treatment of the signs and symptoms of DED in adult patients. Shire began to launch Xiidra in the United States in the third quarter of 2016.
Second, patients suffering with keratoconus received good news with the FDA’s approval of Avedro’s Photrexa Viscous, Photrexa, and the KXL System. Photrexa Viscous and Photrexa are photoenhancers indicated for use with the KXL System in corneal collagen cross-linking (CXL) for the treatment of progressive keratoconus. This past fall saw the first procedure performed in the United States, and we anticipate the number of procedures performed to grow in the upcoming year.
Third, on the legislative front, there has been much debate this past year about in-office versus online eye exams. Georgia passed HB775, which ensured that people can only receive a glasses or contact lens prescription after they have had an in-person eye exam with an optometrist or ophthalmologist. Additionally, in South Carolina, following Gov. Nikki Haley’s veto of S. 1016—the Eye Care Consumer Protection Law—both chambers of South Carolina’s General Assembly approved a countermand that effectively sends the bill to the Secretary of State’s office to become law. Jointly supported by optometry and the South Carolina Medical Association, S. 1016 mandates that eyecare kiosks and other similar technologies be held accountable and provide the same standard of care expected of a qualified and competent eyecare physician. Several other states, including Alabama, Indiana, Maine, Michigan, Mississippi, Nebraska, Ohio, and West Virginia, have enacted similar safeguards and laws.
Along these same lines, online vision tests that prompt individuals through a series of charts on their smartphone and home computer continue to be in the crosshairs of several major optometric and ophthalmic associations.
Lastly, there has been one area that we’ve been talking about for years, and we keep predicting that “next year” with be the year for it—myopia control. As noted earlier, this year’s Reader Profile Survey results show an increase in the number of practitioners using myopia control with contact lenses of 13% over 2015. Couple that jump with myopia control workshops—such as “Controlling the Progression of Myopia: Contact Lenses and Future Medical Devices,” which was cosponsored by the FDA, American Academy of Ophthalmology, American Academy of Optometry, American Association for Pediatric Ophthalmology and Strabismus, American Optometric Association, American Society of Cataract and Refractive Surgery, and Contact Lens Association of Ophthalmologists, Inc.—and 2017 may be the year in which currently off-label myopia control methods become on-label. CLS
Special thanks to Deborah Fisher and Lisa Starcher for their help in writing this article.
For references, please visit www.clspectrum.com/references and click on document #254.
Dr. Nichols is an assistant vice president for industry research development and professor at the University of Alabama-Birmingham as well as editor-in-chief of Contact Lens Spectrum and editor of the weekly email newsletter Contact Lenses Today. He has received research funding from Johnson & Johnson Vision Care and honoraria from Shire.




