Contact Lenses for the Irregular Cornea
Loretta B. Szczotka OD, MS
JUNE 1998
If managed appropriately, patients with irregular corneas can enjoy clear vision and successful contact lens wear.
In optometry school, I learned the official definition of abnormal irregular astigmatism according to the Dictionary of Visual Science: astigmatism in which the two principle meridians of the eye are not at right angles to each other due to abnormalities of the cornea caused by injury or disease. With this, I envisioned the typical topographic bow tie form of corneal astigmatism, with the two principle meridians at oblique angles to each other, as commonly occurs after penetrating keratoplasty (PK) (Fig. 1).
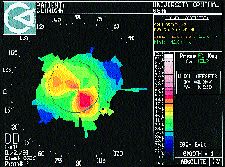
FIG. 1: Post-PK asymmetric irregular astigmatism with two steep
hemi-meridians that are not 180 degrees apart.
When I entered practice, however, this definition was not sufficient for me to explain the visual loss of patients diagnosed with subtle corneal conditions such as rosacea keratitis, herpes simplex stromal keratitis, anterior basement membrane dystrophies and all the other classic conditions known to induce irregular corneal astigmatism (Table 1). Many of these conditions induce focal epithelial and stromal abnormalities which disrupt the regularity of the anterior refracting corneal surface and induce excessive light scatter -- another form of irregular astigmatism. This is perhaps best illustrated by modern corneal topography instruments which include statistical indices and maps that describe and quantify corneal irregularity. For example, an irregularity map on the Humphrey Atlas System displays local elevation differences between the actual corneal surface and the best fit computer-generated regular toric surface (Fig 2). This type of display is helpful in describing the visual limitations of the ocular surface that may not be due solely to obliquely incidenced astigmatic meridians.
| TABLE 1: Corneal conditions that induce irregular
astigmatism (*discussed in body of text) CORNEAL DYSTROPHIES CORNEAL DEGENERATIONS: OTHER CORNEAL PATHOLOGY SURGICALLY INDUCED |
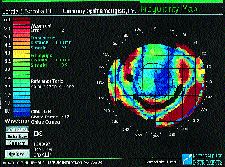
FIG. 2: Irregularity map showing irregular astigmatism and deviations from
a best fit toric surface (post-ALK patient).
Although patients may be reluctant to pursue contact lens fitting after trauma, refractive surgery, or after they have just been diagnosed with a corneal disease, contact lenses are an efficient and safe method of treatment. The corneas of these patients are no longer a simple toroidal refracting surface, so spherocylindrical spectacles cannot fully correct the resultant irregular astigmatism. Rigid contact lenses are an ideal option because they can eliminate the irregularity of a refracting corneal surface.
Never assume that you can't help a patient who has an irregular cornea until you've placed a contact lens on his eye and performed a spherocylindrical overrefraction. Fitting the irregular cornea entails knowing all contact lenses, fitting techniques and multiple presentations and complications secondary to a particular disease or surgical technique. Finding the optimal contact lens design is challenging yet rewarding because these patients often have no other visual option and may be functionally dependent on the contact lenses that you prescribe.
Contact Lenses for Anisometropia
In addition to irregular astigmatism, anisometropia that exceeds amounts found in the normal patient population can exist in many of the corneal conditions listed in Table 1. Anisometropia can be refractive or axial, but corneal-induced anisometropia is almost always refractive. Spectacle correction in refractive anisometropia results in an exaggerated image size differential which can induce secondary aniseikonia. The symptoms and potential for aniseikonia are proportional to the image size differential between the patient's eyes. To predict the amount of magnification differential, apply one percent magnification per diopter of refractive difference between the patient's eyes. Greater than a five-percent magnification difference usually results in impaired or absent binocular vision, and it is not uncommon in corneal disease for this much spectacle corrected image differential to exist. Therefore, contact lenses are often the preferred treatment.
Epithelial and Stromal Irregularities
Conditions like anterior basement membrane dystrophy (ABMD), herpes simplex keratitis and healed ulcers with residual scarring present with epithelial or stromal areas of corneal irregularity. If the affected area extends into the visual axis, spectacle corrected visual acuity will be compromised.
Anterior basement membrane dystrophies typically present with diffuse corneal irregularity. In ABMD, the mechanical friction of an RGP lens can cause epithelial erosions. Firm hydrogel lenses or lenses with a center thickness greater than that of typical thin disposable lenses are best to prevent conformation to the irregular corneal contour. I often choose either a prism ballasted sphere or toric such as the Hydrasoft daily wear lens (CooperVision) or a non-HEMA material such as the CSI Clarity sphere or toric (Wesley Jessen) because they can mask mild corneal irregularity in ABMD disease. Patients with ABMD who have better than 20/40 spectacle acuity are the best candidates for soft lenses. When spectacle corrected acuity is worse than 20/40, try RGPs. With RGPs, aim for an apical clearance lens-to-cornea relationship to prevent mechanical friction and corneal erosions. A moderate to high Dk RGP material helps preserve the integrity of the corneal epithelium and stroma. Routine protein removal or use of a strong emulsifying surfactant such as that used in the Claris system (Menicon USA) is recommended to solubilize deposits which can increase the friction of a high Dk RGP lens if not removed.
Herpes keratitis and healed ulcers and lacerations with residual scarring usually present with abrupt zones of corneal irregularity which can induce diffuse corneal topographic changes and irregularity distant from the affected area (Fig 3). RGPs are usually required. Scarred herpetic keratitis and healed ulcers often leave residual depressions which will pool with fluorescein when RGP lenses are applied (Fig. 4). Healed lacerations will usually create a flattened area over the scar, similar to an RK incision, which can act as an elevated pivot point during RGP lens fitting. When fitting RGPs, attempt to vault the injured or diseased area, although doing so without excessively impinging on adjacent healthy tissue may be difficult because the corneal contours and resultant fluorescein patterns are atypical and asymmetrical. Moderate to high Dk RGPs are recommended to preserve the already compromised tissue
.
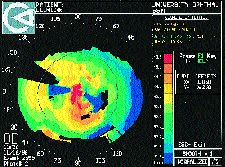
FIG. 3: Patient with residual scarring from a corneal ulcer. Note local
flattened zones with irregularity in visual axis.
FIG. 4: RGP fluorescein pooling over a zone of irregularity following
herpes keratitis.
Keratoconus
The treatment of corneal irregularity induced by keratoconus has long been associated with rigid contact lenses, although no standard method of lens fitting exists. Possible lens-to-cornea fitting relationships include apical clearance, divided support/three-point touch or apical corneal touch, which may have minimal peripheral stabilization. It's important to classify the degree of apical corneal bearing due to a possible association with apical scarring. The only prospective randomized trial to attempt to associate the fitting relationship with scarring was performed by Korb, et al., in 1982. In this seven-patient, randomized trial, one eye was fit with central touch and the other eye was fit with a central clearance relationship. After 12 months, scarring occurred in four of the seven eyes fit flat, and no scarring occurred in the steep-fit group. Due to the small sample, the results were inconclusive about whether contact lenses influence the disease course directly.
In addition to tremendous variability in the techniques and lenses used to fit keratoconus patients, there are no uniformly accepted baseline data points to apply toward initial lens selection. I routinely use corneal topography both qualitatively and quantitatively instead of keratometry prior to trial lens selection. Initially, I assess the morphological shape of the cornea based on an instantaneous (tangential) topographic display. Usually the cone type can be classified as either a central (nipple) cone or a sagging (oval) cone (Figs. 5 & 6). In a central cone, the area of steepening is located very near the visual axis, is small in diameter and is uniformly surrounded by corneal flattening. A sagging cone is characterized by a larger area of steepening located usually infero-temporally to the visual axis, and may have a thinner zone of flattening peripheral to the cone in the same quadrant as the cone dislocation. My fitting philosophy varies based upon the cone morphology (Table 2).
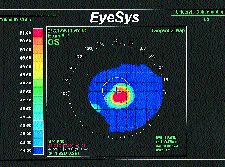
FIG. 5: Tangential map demonstrating a central (nipple) cone in
keratoconus.

FIG. 6: Tangential map demonstrating a sagging (oval) cone in keratoconus.
| TABLE 2: Keratoconus fitting method based on cone
morphology CENTRAL CONE: SAGGING CONE: |
For a quantitative topographic approach to RGP fitting, I utilize the axial (sagittal) maps to choose an initial trial lens. A recent retrospective study of keratoconus patients performed at University Hospitals of Cleveland revealed that certain topographic data points were predictive of an RGP lens fit with faint apical touch. For all patient groups, the axial steep simulated keratometry reading (SIM-K) was best in predicting an RGP base curve that achieved minimal apical bearing. However, in a decentered cone subgroup, the curvature at the peak of the apex on an axial map was the most predictive of an RGP base curve that achieved a minimal apical touch fit. Therefore, I either begin with the axial steep SIM-K or the axial apex curvature when choosing an initial diagnostic lens for centered and sagging cones, respectively, if attempting a minimal apical touch fit.
Corneal Degenerations
The irregular astigmatism created in Salzmann's nodular degeneration and Terrien's marginal degeneration is managed well with RGPs. Spectacle corrected acuity deteriorates in Terrien's marginal degeneration secondary to increasing irregular astigmatism formed by progressive thinning of the peripheral cornea. Salzmann's nodular degeneration is characterized by elevated subepithelial nodules in either a scarred cornea (Fig. 7) or at the edge of a transparent cornea. The patient in Figure 7 had "count fingers" best corrected spectacle acuity, but achieved 20/30 with a spherical RGP lens. Although keratometry usually reveals high amounts of astigmatism in Salzmann's degeneration, bitoric lenses are usually not indicated because the irregularity is diffuse and topography reveals asymmetric and unequal curvature variations along a given meridian (Fig. 8).
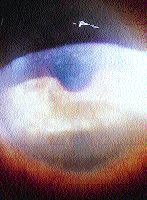
FIG. 7: Salzmann's nodular degeneration

FIG. 8: Topography of patient in Fig. 7. Note astigmatism is asymmetric and
irregular.
Penetrating Keratoplasty
Irregular corneal astigmatism occurs in an estimated 25 percent of eyes after penetrating keratoplasty (PK) due to surgical technique and patient healing responses. Historically, graft failure and rejection have been the most common complications after penetrating keratoplasty, but with advances in corneal preservation techniques, surgical techniques and postoperative medications, PK patients now enjoy success rates for graft survival as high as 77 to 100 percent several years postoperatively. The most common complications now include regular and irregular postoperative astigmatism and anisometropia, which limits spectacle wear. RGPs are often the best possible option. The full-time use of soft lenses in post-keratoplasty eyes can cause neovascularization and rejection of the donor tissue. Since the cornea is avascular and immunologically privileged, if neovascularization develops in the transplanted eye, immune host mechanisms may enter the tissue and cause rejection.
The topography of the cornea after penetrating keratoplasty has been described by Waring, et al. (1992), as one of five major shapes: prolate; oblate; mixed prolate and oblate; asymmetric; and steep-to-flat. The prolate shape is steeper in the center and flatter in the periphery, similar to the asphericity of the normal human cornea. On a standard topographic color map, this cornea commonly features a symmetric astigmatic red bow tie pattern. The oblate shape is flatter in the center and steeper in the periphery (Fig. 9), and it features regular central astigmatism described as a symmetric blue bow tie pattern. The mixed prolate and oblate shape is described as a regular astigmatic pattern of a red bow tie intercepted by a blue bow tie. In the asymmetric pattern, the distinguishing feature is two steep hemi-meridians which are not 180 degrees apart (Fig 1). In the steep-to-flat pattern, the steepest hemi-meridian is located 180 degrees from the flattest hemi-meridian of the cornea.
.
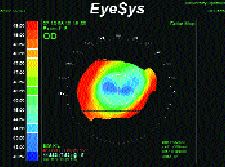
FIG. 9: Oblate corneal shape following a penetrating keratoplasty.
After a preliminary classification of graft shape, select the RGP lens design. Table 3 lists design recommendations for the five topographic patterns found in PK patients. Visually, most eyes do well with spherical rigid lenses because residual astigmatism will rarely be present if most of the corneal astigmatism was surgically induced. However, front surface toric or cylindrical power effect bitoric RGP lenses are occasionally required. The prolate shape can be fit with standard RGP lens designs or a keratoconic design if the central steepening is excessive. The oblate shape is best fit with one of the reverse geometry lens designs which feature a plateau shape with secondary curves 2.00D to 8.00D steeper than the central posterior curve of the optical zone. If the astigmatism in the prolate or the oblate shape is minimal and localized to the central 5.0mm of the graft, it may be possible to vault the entire astigmatic area with a standard spherical lens design. In a mixed prolate and oblate graft, a bitoric lens design is best. Due to the asymmetry of the steep-to-flat and asymmetric patterns, it may be difficult to center an RGP, so large overall and optical zone diameter lenses may be required to help in centration and pupillary coverage. Aspheric lenses may improve centration, but if an aspheric lens remains decentered, visual acuity may be diminished secondary to eccentric viewing through the aspheric periphery.
| SHAPE | SUGGESTED RGP DESIGNS |
| PROLATE | Aspheric Bi-Aspheric Bitoric Keratoconus Design Standard Spherical Posterior Curves |
| OBLATE | Reverse Geometry Lens Bitoric |
| MIXED | Bitoric |
| ASYMMETRIC | Standard Spherical Posterior Curves Keratoconus Design Large Diameter and Optical Zone in case of decentration |
| STEEP TO FLAT | Large Diameter and Optic Zone in case of decentration Standard Spherical Posterior Curves Aspheric Periphery |
Piggyback and hybrid lenses have been suggested as methods of addressing postoperative contact lens discomfort and lens decentration, but they should be used with caution for full-time wear because of limited corneal oxygenation. Recently, scleral lenses have been redesigned in high Dk RGP materials. Although currently not available to most U.S. clinicians, they have the potential to visually rehabilitate the difficult to fit corneal transplant patient.
Keratorefractive Procedures
Clinically significant irregular astigmatism may occur after refractive corneal surgery, particularly after radial keratotomy, although it is becoming less common. The majority of my patients who present for contact lens correction after refractive surgery are those who have had RK when it was popularized in the early 1980s. Most are now presbyopic, and many are symptomatic from overcorrections and hyperopic drifts since their original procedure. Irregular astigmatism after RK can result from an eccentric optical zone, uneven healing, unintentional incisions across the visual axis, micro- or macroperforations or intersecting radial and tangential incisions. Irregular astigmatism after PRK is either a result of a decentered ablation, persistent central islands, and rarely corneal stromal haze. Following LASIK or ALK, irregular astigmatism is most often secondary to cap misalignment or loss, epithelial ingrowth or corneal perforation if the microkeratome was not properly assembled (Fig. 10).
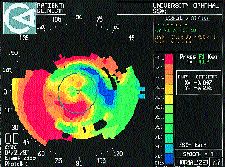
FIG. 10: Asymmetric irregular astigmatism in the optic zone and abrupt
curve changes around the flap (post-ALK).
When fitting RGP lenses after RK, PRK, ALK or LASIK, the contact lens will vault the flatter central cornea resulting in significant fluorescein pooling and a plus powered lacrimal lens. This plus powered tear layer must be compensated with additional minus power in the RGP lens, ironically resulting in a lens power similar to the patient's preoperative sphere power. For standard RGP designs, select a base curve similar to the curvature at the bend of the surgical transition zone on an axial computerized videokeratograph. This should produce a lens with midperipheral corneal alignment and adequate, but not excessive, central pooling. When a reverse geometry design is indicated because of excessive peripheral clearance with a standard peripheral curve system, topography is critical to measure the midperipheral cornea and the curvature changes across the knee of the transition zone. This measurement is facilitated by moving the interactive cursor on topography instruments across the bend of the surgical optical zone to calculate the dioptric change across the transition.
Although soft lenses can be fit for residual regular ametropia following a keratorefractive procedure, RGPs are required to correct significant corneal irregularity. The Harrison Post Refractive Lens (Paragon Vision Sciences) is a soft lens approved by the FDA for postsurgical correction of ametropia. It functions like a reverse geometry design, allowing the central optical portion of the lens to be flatter than the midperiphery, similar to the contour of the cornea. Reverse geometry RGPs after refractive surgery typically require secondary curves 3.00D to 5.00D steeper than their central curves. I use these lenses mostly to manage lens decentration after RK due to isolated steep areas surrounding the RK optic zone. Since RK produces a significant alteration of both peripheral and central corneal topography, a reverse geometry design, which better conforms to the relatively steeper corneal periphery, is often beneficial. Reverse geometry lenses are rarely required after PRK because of the smooth transition from the ablation zone to the unaltered corneal periphery, but they may be required after ALK and LASIK because of more abrupt curvature changes surrounding the corneal cap from use of the microkeratome.
To receive references via fax, call (800) 239-4684 and request document #37.




