ADHERENCE
Physical Chemistry Explains Contact Lens Adherence
By GARY R. BELL, OD, MSEd
November 1998
Contact lens adherence is not a random, unexplainable phenomenon. Understanding some basics of physical chemistry can help you avoid this clinical nuisance.
Contact lens fitters are increasingly becoming aware of contact lens adherence, a particularly common problem in soft and RGP lens extended wear situations. One study reports that all of 20 soft lens extended wear patients examined showed signs of adherence when their eyes were closed with their lenses on. In most cases, the lenses showed signs of immobilization within 45 minutes of eyelid closure (Bruce & Mainstone, 1996). Helen Swarbrick, Ph.D., and Brien Holden, Ph.D., estimate that 40 to 50 percent of extended wear RGP patients experience lens adherence. Robert Campbell, M.D., and Patrick Caroline, F.A.A.O., estimate that 80 percent of extended wear RGP patients experience adherence on some occasions. After evaluating 15 RGP daily wear patients having a tendency for adherence, Drs. S. Barry Eiden and Cristina Schnider described characteristic clinical signs (Table 1).
These investigators support Swarbrick's theory of adherence mechanism -- that the post-lens tear film thinning and increased viscosity of the post-lens film causes lens mobility to decrease until it becomes adhered. In this paper, I don't disagree with what Swarbrick suggests, but I look more deeply into the physical chemistry factors at play.
Adhesion Theory
Why do any two objects stick together? In the realm of physical chemistry, the most widely accepted theory of adhesion is the wettability-absorption theory. This says that an adhesive must come into complete, intimate contact with an adherend in order to stick to it -- a state described as "completely wetting the adherend." At this state, intermolecular attractions (also known as van der Waals forces), dipole-dipole interactions and a number of other electrostatic forces are acting at or near their maximum capacity.
Once the two bonding surfaces are thoroughly wetted, the liquid adhesive must pass through a transition phase to become a tough, nonliquid, load-bearing interlayer. This change in state can be accomplished by simple evaporation, but can be greatly enhanced if a slow crystallization of components occurs in the interlayer (Fig 1). Observers have noted a fern-like fluorescein pattern when an adhered RGP lens is separated. Tear ferning experiments have confirmed crystallization of the tear film mucin with dehydration. Whether the retro-lens debris ring of an adhered RGP lens is best characterized as a crystallization or as an amorphous precipitate is yet to be determined (Fig. 2).
Adhesion increases with closeness of contact. Bruce and Mainstone (1996) demonstrated that adherent soft lenses were in close enough contact with the cornea to produce thin film color interference phenomena in the post-lens tear film as viewed by biomicroscopy with specular reflection. The topography of the adhering surfaces is another determining phenomenon at work in adherence situations. Smooth and parallel surfaces, such as the cornea and a contact lens, allow closer, more intimate contact of the surfaces, which facilitates adhesion. The curved surfaces of the cornea and lens increase the relative area of contact, increasing adhesive forces. The closed upper lid also contributes an adhesion force by providing a continuous inward push of the contact lens toward the cornea.
Intermolecular Attractions
Atoms and molecules exhibit attractive forces to other atoms and molecules that are within a one-nanometer (nm) distance, though the distance varies somewhat with different substances. Within this attraction zone, the attractive forces increase by the reciprocal of the distance to the sixth power (1/d6). When the distance closes to about 0.3nm, the intermolecular forces reverse to become a powerful repellent force. Figure 3 demonstrates the force plot of a typical material. Explained by quantum mechanics, intermolecular attractions occur essentially because electrons don't like to be confined to the same small space. The rapid motion of electrons causes instantaneous fluctuations in the charge density around the nucleus. These charge fluctuations produce an overall small electrical charge, which causes the atom or molecule to attract and orient toward the opposite charge area of another molecule. Although these intermolecular forces are capable of providing adhesion, it seems unlikely that the contact lens would often come within one nanometer of the cornea. Other forces must be considered.
Dipole-Dipole Interactions
The forces of intermolecular attractions are greater when there are dipoles, according to Richard Feynman, author of The Feynman Lectures on Physics. Water is a classic example of a dipole molecule (Fig. 4). Describing the molecule, Feynman says, "... the negative charges sit more on the oxygen, and the mean positions of the negative charges and of the positive charges are not at the same point, consequently, another molecule nearby feels a relatively large force." As an attractive force between two surfaces, the dipole-dipole forces of water molecules in the tear film adds to the intermolecular forces to help form an adhering force. Intermolecular bonds with a dipole moment are sometimes called a hydrogen bond.
The Dry Eye Connection
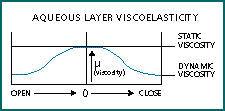
As noted earlier, Drs. Eiden and Schnider found that all 15 of the patients they had identified as prone to RGP daily wear lens adherence showed evidence of dry eye. Dry eye is characterized by a thinning of the tear film and increased tear viscosity. Tear film thinning moves the lens closer to the cornea, increasing the distance-sensitive adhesion forces of intermolecular attractions and dipole-dipole interactions. The excessive tear evaporation of many dry eyes causes the tear film to become hypertonic, which increases the viscosity of the tear film. Increased viscosity increases resistance to movement. The aqueous layer of the tear film also demonstrates variable viscosity with shear thinning. This means that the stasis of the closed eye environment causes glycoproteins in the tear film to form weak connective fibrils, which can cause a five- to six-fold increase in viscosity (Fig. 5).
Adhesion Molecules
Expanding knowledge of the immune system has led to the identification of molecules associated with physiological adhesion. Known adhesion molecules include the integrins, the immunoglobulins, the cadherins and the selectins, although others are rapidly being discovered. Adhesion plays an important early role in the immune-related complement cascade. Once C3b protein is activated, either by an antigen-antibody reaction or by bacterial proteins, it adheres to the foreign invader and continues the cascade of proteins that promote acute inflammation. This C3b coating process is an example of immune adherence or opsonization. C3b proteins can bind to foreign membranes by covalent bonds, while immunoglobulins connect to foreign agents using crystallization .
A serology study found high levels of cytokines (adhesion molecules) expressed by the epithelial cells of the salivary ducts of patients with Sj�gren's Syndrome. Swarbrick and Holden have noted the preponderance of inflammatory cells in the closed eye tear film, which may mean that many adhesion molecules may be present in the closed eye situation. It is not known whether adhesion molecules play a role in lens adherence. If they do, it may be in providing a collective tack or stickiness to the lens surface.
Preventive Tactics
There's probably greater interest in finding solutions to lens adherence problems than in learning about adherence mechanisms, but understanding the causative mechanisms can enlighten us on how to prevent contact lens adherence. Here are a few suggestions.
Avoid extended wear. Extended wear is often a precursor to lens adhesion. It results in more intimate contact, constant lid pressure, tear film thinning and increased tear viscosity. A sensible policy is to avoid extended wear in any patient that shows corneal staining with sequential fluorescein staining, or to avoid it all together.
Change the base curve. When RGP adherence occurs, change the base curve of the offending lens. Based on my experience, it seems a 0.50D change is necessary to make a significant reduction in adherence tendencies. Trial fit a lens that is 0.50D flatter than the offending lens. If the movement is not too excessive, that base curve should be a suitable substitute. If the movement with the flatter lens is excessive, try a trial lens that's 0.50D steeper than the original lens, with increased secondary blending and a higher Dk material.
Treat the dry eye. Use any available method such as lid scrubs, artificial tears and punctal occlusion. Increasing the depth of the tear film greatly reduces distance-sensitive adherence factors such as intermolecular attractions and dipole-dipole interactions.
Reduce RGP lens size. This reduces the area of potential adhesion. Weigh this approach against other factors, such as 3 and 9 o'clock staining, which may increase with an interpalpebral fit in a dry eye.
Rehydrate soft lenses. Instruct patients with soft lens adherence to thoroughly rehydrate their lenses before attempting to remove them. Partial adherence can be a precursor to lens tearing. Consider a refit with a flatter soft lens.
Institute more effective lens cleaning techniques. The retro-lens debris ring seen in many cases of RGP lens adherence may indicate chronic adhesion problems, with the deposition of adhesion-promoting materials posing a real threat to successful lens wear. New daily enzyme cleaners may be a helpful addition to the lens cleaning regimen for preventing contact lens adhesion.
Putting it All Together
Contact lens adherence can be explained by known characteristics of physical chemistry. This paper supports Swarbrick's theory that tear film thinning and increased viscosity are precursors to lens adherence, but I have elaborated on the mechanistic details. The constant lid pressure present in extended wear situations is a factor in expressing fluid from under the lens. Wetting of the surfaces, present in the post-lens environment, is a necessary step for adherence, according to adhesion theory. Topographical factors, such as smoothly polished, parallel surfaces, increase the likelihood of complete surface wetting. The transition of the wetting liquid into a tough, nonliquid, load-bearing interlayer is facilitated by tear evaporation and possibly tear component crystallization. Tear ferning experiments provide further evidence of the crystallization of tear film components. The electrostatic forces of intermolecular attractions and dipole-dipole interactions provide the ongoing forces holding the adhered lens to the eye.
The connection between dry eye and adhesion seems to be reinforced by physical factors. Swarbrick and Holden have shown that with decreased tear production in the closed-eye state, most people have dry eye in the extended wear situation. Adhesion molecules may play a role in the adhesion of contact lenses, particularly in the dry eye state. Dr. Silbert strongly connects lens adhesion to contact lens related corneal ulceration. Many bacteria, such as Pseudomonas aeruginosa, must adhere to a human cell as the first step to pathological colony formation. It seems logical to infer that adherence of a contact lens to the eye may enhance the ability of a bacterium to adhere to the cornea to start replication. It is also possible that lens adhesion, by preventing tear flow, prevents immune defense cells from moving to the point of injury.
Considering the information presented here, it seems that preventing lens adherence involves avoiding extended wear until less adherent lenses are developed, changing base curves, treating the probable dry eye situation, decreasing RGP size if feasible and, in the case of soft lenses, rehydrating the eye thoroughly before removing the lens. Additional measures to ensure the effectiveness of lens cleaning are also recommended. CLS
References are available upon request to the editors at Contact Lens Spectrum magazine. To receive them via fax, call (800) 239-4684 and request document #42.
Dr. Bell, formerly an instructor at the Southern California College of Optometry, has published numerous articles on myopia and contact lenses. He is currently in private practice in Corona, Calif.

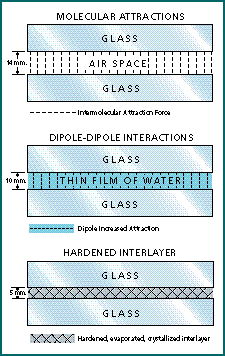

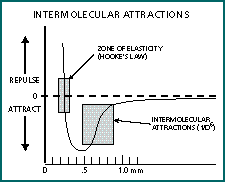
TABLE 1
Signs of adherence in RGP patients
-Signs of tear deficiency using sequential fluorescein staining
-Irregular, retro-lens debris ring in the midperiphery in most cases
-A corneal indentation ring adjacent to the edge is always present
-Central corneal staining is often present following lens removal
-Peripheral arcuate staining is present outside the zone of adherence
From: Eiden, SB, Schnider, C., "Adherence of Daily Wear RGP Contact Lenses," Contact Lens Spectrum, 2: 42-46, 1996.