Which Products Keep Deposits Under Control?
David Keith, Ph.D., Jerry Stein, Ph.D., FAAO, Mike Christensen,
O.D., Ph.D., Ralph Stone, Ph.D., Darell Turner, Ph.D., Susan Luna, M.S.,Leslie Napier,
Ph.D.
AUGUST 1999
These five lens care regimens battled protein deposition head-on. Find out which ones came out on top.
Differences in the cleaning effectiveness of lens care regimens have been associated with variations in patient satisfaction, comfort and visual performance levels. One method of evaluating the cleaning efficacy of a regimen is to measure the amount of protein deposition left on a patient's lenses after a specified wearing time. Protein deposits begin to form on lenses immediately following insertion, increase with time and are generally recognized as a contributing factor to the increased incidence of ocular signs and symptoms as lenses age. Despite the growing popularity of frequent replacement schedules, the speed of lens deposition remains a significant concern for practitioners due to its potential impact on patient comfort and visual performance.
About Lens Cleaning Products
Lens cleaning products, such as daily cleaners and multipurpose solutions (MPS), contain various components that aid in the removal of protein deposits from contact lenses. One of these components, citrate, acts by interacting with cationic protein molecules, including lysozyme, to displace deposits from the lens. Lysozyme is a major component of the tear proteins that are associated with the film-like deposits on lenses. In addition to removing deposits during the rub and rinse process, citrates have been shown to have "passive cleaning" action, which means that they continue to aid in deposit removal even while lenses soak.
This report presents findings from three studies that evaluated the effectiveness of four popular lens care regimens and one new regimen in minimizing protein deposition on contact lenses worn between 30 and 90 days.
Methods
Four-hundred and thirty patients enrolled in three independent studies at 13 sites in the United States*. The studies were randomized, investigator-masked designs and all patients were healthy, successful contact lens wearers. Three of the five regimens tested contain citrate.
Citrate-containing test regimens included: 1) the New Opti-Free Express Multi-Purpose Disinfecting Solution (MPDS) with Aldox (introduced to the U.S. market in May, 1999); 2) Opti-Free formulation** alone; and 3) Opti-Free formulation** in conjunction with Opti-Free Daily Cleaner. Non-citrate-based regimens included: 1) ReNu MPS and 2) ReNu MultiPlus MPS.
All patients wore one pair of FDA Group IV contact lenses on a daily wear basis for the duration of the studies (Studies 1 and 2 were conducted over a 90-day period; Study 3 over a 30-day period). Lens care products were used according to their individual package inserts.
Calculating the Results
Investigators collected the lenses at the completion of the study and returned them to the sponsor within approximately 24 hours. Lenses were placed into an extraction solution which removes greater than 95 percent of the lysozyme from Group IV ionic lenses. The resulting extraction solution was then analyzed by HPLC to determine the total lysozyme removed from the lenses. The HPLC procedure is a reliable method with a relative standard deviation of approximately 2 percent. Technicians conducting all HPLC analyses were masked as to which treatment regimens they were testing.
Study Findings
Lysozyme levels were consistent for Studies 1 and 2, demonstrating the reliability of the HPLC technique to measure residual lens lysozyme. In addition, the results of the studies consistently demonstrated that citrate-containing regimens are significantly more effective than non-citrate regimens in minimizing lysozyme deposits (Figs. 1 and 2). Lysozyme levels associated with the two non-citrate regimens (ReNu and ReNu Multi-Plus) did not differ substantially from each other. The superior performance of the citrate-containing regimens may be attributed to the ability of citrate to aid in deposit removal during the rub and rinse step as well as during a passive soak.
Other studies have shown that the daily use of a separate pancreatin-based daily protein remover, such as Opti-Free SupraClens (Alcon), further enhances the effectiveness of the Opti-Free formulation by reducing lysozyme build-up by approximately 50 percent more than using the Opti-Free formulation alone.
Pancreatin, the active ingredient in SupraClens, is an enzyme. Note that none of the MPSs tested in these studies contain an enzyme. Thus, additional benefits can be obtained by using this separate daily protein remover.
Daily use of the new Opti-Free Express MPDS with Aldox for 30 days (Study 3, Fig. 2) resulted in substantially less lysozyme build-up than with the daily use of the other multi-purpose solutions. This lower residual lysozyme can perhaps be attributed to the unique combination of a citrate and surfactant system in this new MPDS. The New Express MPDS with Aldox has also been shown to provide superior anti-microbial activity without compromising patient comfort or ocular health.
Results of these studies indicate that formulation differences between lens care regimens can significantly impact the amount of protein deposits that accumulate on contact lenses. These differences in product performance may impact patient comfort, visual performance and ocular health and thus, bear important implications for practitioners who recommend lens care products to their patients.
*Participating Investigators: Peter Bergenske, O.D.; Robert Bevington, O.D.; Ronald Cedrone, O.D.; Bobby Christensen, O.D.; Douglas Cox, O.D.; Frank Fontana, O.D.; James Key, M.D.; Kenneth Lebow, O.D.; Gary Meier, O.D.; Lee E. Rigel, O.D.; Craig Smith, M.D.; Mary Jo Stiegemeier, O.D.; and Eric White, O.D.
**Marketed as a multipurpose solution under the trademark Opti-Free Express Multi-Purpose Solution and marketed when used with a daily cleaner under the trade name Opti-Free Rinsing, Disinfecting and Storage Solution.
References are available upon request to the editors at Contact Lens Spectrum. To receive references via fax, call (800) 239-4684 and request document #51. (Be sure to have a fax number ready.)
All seven authors work for Alcon Laboratories. David Keith is associate director of
Analytical Chemistry-Consumer Products. Jerry Stein is director of Consumer Products
Clinical R & D. Mike Christensen is manager of Clinical Research-Consumer Products.
Ralph Stone is vice president of Consumer Products R & D. Darell Turner is director of
Biometric Sciences. Susan Luna is a biostatistician in Biometric Sciences. Leslie Napier
is a senior clinical research scientist-
Consumer Products Clinical.
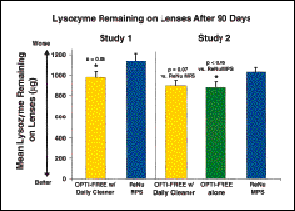
FIG. 1: Residual lens lysozyme following 90 days of wear. Study 1: n = 39 and 35
evaluated patients for Opti-Free with Daily Cleaner and ReNu, respectively. Study 2:
n = 44, 39 and 42 evaluated patients for Opti-Free with Daily Cleaner, Opti-Free formula
and ReNu, respectively. All values are means � S.E
.
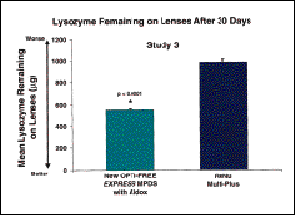
FIG. 2 (Study 3): Residual lens lysozyme following 30 days of wear. n = 89 and 88
evaluated patients for Opti-Free Express MPDS with Aldox and ReNu Multi-Plus,
respectively. All values are means � S.E.



