Naso-Lacrimal System Occlusion for Dry Eye
A review of the etiology and differential diagnosis of ocular surface disease, with emphasis on occluding the naso-lacrimal drainage system as a treatment.
BY CLIFFORD SCOTT, OD, MPH, & PAUL WHITE, OD
FEBRUARY 1999
The increasing number of older individuals in the population is bringing about a higher prevalence of symptomatic, age-related eye problems, including ocular surface disease (OSD). Its most common presentation, tear-deficient dry eye, is relatively easy to distinguish from the other types. Home remedies and over-the-counter preparations sufficiently alleviate mild symptoms, but patients who have more recalcitrant forms require a thorough differential diagnosis to isolate the causes. A comprehensive treatment plan not only directs attention to relieving the tear film insufficiency, but also addresses any associated conditions that may aggravate the symptoms or prevent resolution of the underlying problem.
Pre-Ocular Tear Film
The normal secretion and distribution of tears is essential for clear and comfortable vision. The tear film forms a smooth surface over the optically uneven corneal epithelium, provides lubrication for eyelid movement, acts as a nutrient vehicle for ocular surface metabolites, dilutes irritants, flushes away debris, provides antibacterial activity and transports white blood cells to help heal corneal injuries. These complex and interrelated functions are dependent upon the continuous production of tear components, their segregation into a multi-layered film over the ocular surface and frequent blinking to remove and resurface that tear film. The stabilized pre-ocular tear film (POTF) consists of three functional layers:
The inner mucin layer -- primarily composed of glycoproteins secreted by goblet cells in the surface of the conjunctival epithelium. This layer is at least 1�m thick and functions as the interface between the hydrophobic surface epithelial cells and the aqueous tear layer.
The middle layer -- composed mostly of water with electrolytes, IgA and proteins, some of which have antibacterial enzymatic activity. Secreted by the main and accessory glands, which are both under neural control, it is the main component of basal and reflex tearing. This layer is about 7 �m thick, though recent investigations show that the inner and middle layers may be different manifestations of a single layer.
The lipid phase -- a single molecular layer of cholesterol esters, low polarity lipids, free fatty acids and waxes. This layer is about 0.1 �m thick and floats on the aqueous layer. Produced mainly by the meibomian glands, this layer serves to thicken, stabilize and retard evaporation of the aqueous layer.
Basic secretion, a constant slow flow of tears, is produced by all of the orbital secretory glands. Reflex secretion, an increased rate of tearing caused by neural stimulation, occurs in the main lacrimal gland. However, there is some evidence that all of the glands are regulated and can therefore respond to challenges and changes in the environment, aiding in the maintenance of a stable and constant tear film.
Although a decreased production of basal tears is typically part of the aging process, a concomitant stenosis of the puncta usually results in a normal balance between tear secretion and elimination in older individuals. Even though they're free from dry eye symptoms, older patients often have epiphora during reflex tearing. Others, however, may have consistent symptoms related to inadequacies of their tear layer.
In the Blink of an Eye
The blink has a profound influence on the structure, stability and function of the tear film. In addition to its obvious protective function, blinking smooths the anterior surface of the mucin layer, spreads and thins the lipid layer within the palpebral aperture and possibly stimulates the discharge of meibomian and goblet cell secretions. The sharply edged posterior border of the lid acts as a squeegee in removing depleted tears during the closing phase of the blink, and in applying a fresh layer during the opening phase.
Tears are eliminated from the eye by a pump system which is activated by blinking. Each punctum opens into a short vertical canaliculus which turns nasally into the horizontal canaliculus. The superior and inferior horizontal canaliculi merge into the common canaliculus which opens into a reservoir, the lacrimal sac. Each blink compresses the lacrimal sac, which forces its contents down through the naso-lacrimal duct into the inferior meatus of the nose. Opening the lids re-expands the sac, creating negative pressure within the canaliculi and drawing tears in through the puncta from the lacrimal lake.
Types of Dry Eye
The National Eye Institute/Industry Workshop (1995) has reclassified dry eye (keratoconjunctivitis sicca, or KCS) into two categories: tear deficient and evaporative. However, the condition is evaluated in clinical situations by its symptoms and appearance. Insufficient or abnormal production of any of the three pre-ocular tear film layers or interference with normal blinking can cause signs and symptoms of dry eye. An effective treatment plan can be instituted only when a proper diagnosis of the underlying cause has been made. Consideration has to be given to the epidemiology, the pathophysiology and the clinical presentation of each type.
The most prevalent form of dry eye, decreased lacrimal secretion, is bilateral and most commonly occurs in the fifth decade of life, particularly in women. Because it causes a foreign body sensation, it can give rise to pseudoepiphora, the spurious complaint of watery eyes due to irritative reflex tearing. Slit lamp observable signs include decreased tear meniscus, debris and mucus strands in the tear film and, in severe cases, corneal filaments. Primary Sj�gren's syndrome, the most common cause of keratoconjunctivitis sicca, is an inflammatory disease of the exocrine glands associated with dry mouth and other mucus membranes. It may be a primary autoimmune condition itself or it can be a secondary finding associated with other connective tissue diseases, such as rheumatoid arthritis. It is more common in menopausal females, with 40 to 50 years old being the usual age of onset. It also occurs frequently in women who are pregnant or taking birth control pills whose estrogen and prolactin levels are elevated. Ocular symptoms are often the first manifestation of Sj�gren's syndrome. Other local, systemic and exogenous conditions can also impact lacrimal flow (Table 1).
TABLE 1: Conditions that Impact Lacrimal Production
|
Primary mucin deficient dry eye is rare, although it can result from certain conditions that affect the conjunctiva. A reduction in the number of conjunctival goblet cells, resulting in a decrease in mucin production, can be caused by any condition that is damaging to the conjunctiva, including cicatricial ocular pemphigoid, erythema multiforme (Stevens-Johnson syndrome), severe trachoma or chemical burns, especially alkali.
Lipid deficiency is typically associated with lid disorders such as inflammation, trauma or scarring after eyelid surgery. One of its most common causes is chronic blepharitis. Not only is there a decrease in the production of lipids, but the composition of meibum that is secreted has a low molecular weight, resulting in a tear evaporation rate up to 10 times faster than normal.
Removal of the depleted tear film and its subsequent resurfacing can be affected by anatomic defects of the lid or by impairment of its function. Alteration of the normal lid structure usually results in an irregular mucin layer, while incomplete or infrequent blinking causes excessive tear evaporation and possibly exposure keratopathy. Contact lens wear can produce dry eye symptoms in an individual with a pre-existing marginally dry eye. Not only do these hydrophobic materials require greater surface wetting, but contact lens wear itself thins the pre-ocular tear film and interferes with mucin resurfacing.
Corneal epitheliopathies are characterized by an irregular surface of microvilli that prevent mucin from adhering to the cornea. Corneal scars, chemical burns, recurrent corneal erosions, contact lens wear complications, trauma from entropion, or lash abnormalities such as distichiasis or trichiasis are common causes of corneal epitheliopathies.
Diagnosis
Evaluation of the patient suspected of having dry eye syndrome includes a careful case history, an assessment of associated risk factors and an examination of the anterior ocular structures. A thorough case history emphasizing the patient's symptoms is important. It's essential to assess associated conditions that make an individual more likely to develop tear film abnormalities, including advancing age, rheumatoid arthritis, Grave's disease, the use of medications known to cause decreased tear production, abnormalities of the lid, impaired blinking or a history of lid trauma. The most frequent complaints include burning, itching and foreign body sensation.
An external examination without magnification and a slit lamp examination, especially with fluorescein, show characteristic early changes of the external eye. An overall lack of luster results from diminished reflection off of the corneal and conjunctival surface. Moderate degrees of aqueous deficiency also result in mucus strands, filaments, furrows, dellen staining or punctate keratopathy. Rose bengal dye, which selectively stains devitalized cells, is extremely valuable in highlighting desiccated corneal and conjunctival cells. The pre-ocular tear film itself demonstrates increased viscosity, debris, foamy secretion and a scanty inferior tear prism. The lid margins are often keratinized and crusted.
Tear Quantity Tests
You can confirm the diagnosis of aqueous deficient dry eye using tear quantification testing. Although there are many other clinical tests, the most commonly used include the following.
The Schirmer 1 test, with or without topical anesthesia, measures the amount of tear volume available to saturate a standardized filter paper strip placed in the lower cul-de-sac for five minutes. The normal expected value is 15mm or more of wetting. EagleVision's test has the measurements premarked on the strip as well as a dye that turns blue when moistened.
The phenol red cotton thread test (Zone-Quick) is similar to the Schirmer test, but uses a thin thread for only 15 seconds in order to minimize reflex tearing.
The rate of dilution of fluorescein by the watery component of the tears as observed under ultraviolet illumination can differentiate normal tear flow from diminished tear production. In a dry eye, high concentrations of fluorescein will be retained for a prolonged amount of time.
The height of the tear meniscus along the lower lid margin represents the volume of liquid in the eye. Fluorescein can make the observation easier (Fig. 1).

FIG. 1: Marked keratitis sicca with corneal desiccation staining and
minimal lower lid margin tear prism.42
Tear Stability Tests
Tear stability, while not a direct measurement of aqueous deficiency, can help to differentiate eyes that have secondary causes for their dry symptoms. The following are the most frequently used in-office tear stability tests.
Tear break-up time (TBUT), which uses liquid unpreserved fluorescein to highlight the aqueous phase of the tear film, is a measurement of the time after a blink until the lipid layer breaks down and produces a dry spot in the fluorescein pattern. The standard for TBUT is 10 seconds or longer (Fig. 2).

FIG. 2: Typical appearance of precorneal tear film break-up.
Tear thinning time uses the reflection of mires as a noninvasive method to observe the regularity of the surface of the pre-ocular tear film.
The Keeler Tearscope illuminates the entire corneal surface with shadow-free light. Time is measured from a blink until the reflection of the mire becomes distorted.
Thin film interference patterns in the tears can be observed using specular reflection from the cornea or conjunctival surface if the lipid layer is sufficiently thick and even. Abnormal lipid production alters the appearance of these patterns.
Treatment
Management of a patient with KCS may require consultation with or referral to the patient's primary care physician for treatment of associated systemic diseases. A comprehensive approach to eyelid, tear film, conjunctival and corneal abnormalities is important, along with periodic re-evaluation, since a primary dysfunction among one of these components will often affect the other two. Contact lens wear may pose a threat to the compromised ocular surface. Additionally, subjective success with contact lenses may be affected by complications of tear film deficiency. Conversely, contact lenses may play a role in the management of selected disorders of the tear film and ocular surface.
Most types of dry eye are caused by factors beyond the patient's control, however, specific therapies are designed to reduce symptoms and maintain a normal ocular surface. Long-term success is best achieved by using the minimum treatment required and by taking into account the need for patient cooperation and cost-effectiveness. Environmental sources of symptoms should be identified and eliminated whenever possible.
The following treatments for KCS produce varying degrees of success, depending on the cause and severity.
- Ocular hygiene emphasizing frequent cleaning of debris from the lid margins removes a potential source of pathogens. Normal face washing with attention to the peri-ocular region is sufficient for most people, but commercially available lid scrubs are available for individuals requiring more help. Frequent applications of warm soaks may open inspissated meibomian glands sufficiently to improve lipid production.
- The use of artificial tears attempts to supplement insufficient tear flow with a solution that mimics the pre-ocular tear film in tonicity, pH, retention time and tear meniscus. With chronic use, preservatives may produce adverse effects, including allergic response, delayed hypersensitivity or toxicity. Unpreserved solutions in unit-dose containers avoid this problem but are expensive.
- Ointments contain emollients that dissolve at body temperature and disperse in the tears to provide lubrication and protection. Because of their viscosity, they often blur vision and are reserved for use at bedtime.
- Gels containing polyacrylic acids liquefy on contact with the eye and produce less blurring than ointments.
- Lacriserts are water-soluble hydroxypropyl cellulose pellets that are placed in the lower cul-de-sac. The preservative-free polymer is released into the tears for 12 to 24 hours. Vision may be blurred, and the eye may have a foreign body sensation.
Punctal Occlusion
When surface treatments don't relieve symptoms, or when patient compliance becomes an issue, tears can be retained by blocking the outflow to the naso-lacrimal system using temporary or permanent methods. As with all clinical procedures, it's important to obtain written informed consent prior to insertion. Collagen rods are available from several suppliers in 2.0mm lengths and in diameters of 0.2mm, 0.3mm, 0.4mm, 0.5mm or 0.6mm. EagleVision plugs are also available in a 1.6mm length. The diameter is determined from slit lamp observation of the opening of the punctum and the examiner's experience. Using a jewelers' or an 'H' forceps, one of these dissolvable plugs is inserted into an undilated punctum then pushed gently into the vertical canaliculus (Fig. 3). In cases where the vertical canaliculus is shorter than the plug, the end may spontaneously extrude from the punctum. Keeping gentle pressure on it with the tip of the forceps will soften the buried portion. In about a minute, the exposed end will fit below the punctum ring. The water-soluble collagen swells quickly and restricts tear outflow for up to 14 days. These rods are generally used to predict the degree of relief that more lasting methods of occlusion will produce. One diagnostic scenario involves putting plugs in the lower puncta of both eyes, then examining or calling the patient the next day. Another strategy occludes both the superior and inferior puncta of one eye and has the patient compare the comfort of that eye to the untreated one.
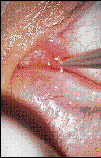
FIG. 3: Inserting a collagen rod into an undilated lower punctum under slit
lamp magnification using an 'H' forceps.
Silicone punctum plugs are a more permanent, yet reversible, method of retaining tears if collagen rod testing is successful (Fig.4). At the present time, there are six companies distributing silicone punctum plugs: Alcon (Tears Naturale), CIBA Vision (Tear Saver), EagleVision tapered shaft, FCI (Ready-Set), Oasis (Soft Plug) and Odyssey (Parasol) (Table 2).

FIG. 4: Diagram of a collapsible tip Parasol silicone plug as it passes
through an undilated punctum.
| Company | Diameters | |||||
| .4 | .5 | .6 | .7 | .8 | 1.0 | |
| Alcon | * | * | * | * | * | * |
| Ciba | * | * | * | * | ||
| Eagle Vision | * | * | * | * | ||
| FCI | * | * | * | * | * | |
| Oasis | * | * | * | * | * | |
| Odyssey | small | medium | large | |||
Each of these distributors offers a set of sizing gauges to determine the proper size plug. If the plug is too small, it may eject spontaneously. A plug that is too large for the punctum will be extremely difficult or impossible to insert. Starting with the smallest size gauge, the amount of resistance is noted as the tip is inserted through the punctum opening. The lid margin should not be distorted forcibly for the gauge to pass through. The proper size gauge is the largest one that fits through the punctum ring with a minimum of restriction (Fig. 5). Pretreating with a drop of topical anesthetic or an anesthetic-soaked pledget may make the procedure more tolerable for the patient and easier for the practitioner.

FIG. 5: Diagram of the proper size gauge as it passes in and out of the
punctum.
Silicone plug insertion can be performed either using appropriate head-borne focusing lenses or the slit lamp. Many practitioners find that the forehead rest of the slit lamp impedes their maneuvering ability. To facilitate insertion, the punctum is usually dilated using either a stainless steel dilator or the plastic dilator that is part of the insertion tool. Lubricating the tip with sterile saline or contact lens wetting solution facilitates its entry. The instrument is inserted approximately 2.0mm vertically, then rotated and inserted another 3.0mm. nasally (Fig. 6). The punctum ring is resilient and will quickly return to its normal diameter, so the plug must be inserted immediately.

FIG. 6: The proper position and motion of the dilator at the beginning of
the procedure.
All plugs come pre-loaded on a stylus with a release mechanism (Fig. 7). The plug is inserted up to its center shaft with the top cap remaining outside the punctum. The release trigger is engaged and the stylus is removed. The top of the plug should be flush with the lid margin (Fig. 8). If it protrudes, gentle pressure with forceps usually seats it properly. In severe cases of dry eye, plugs may be needed in both the superior and inferior puncta, but total occlusion of tear outflow may result in epiphora during reflex tearing.

FIG. 7: Typical release mechanism for a punctum plug stylus.

FIG. 8: Silicone punctal plug in place.
On some lid margins, the punctum is in physical contact with the bulbar conjunctiva. Punctum plugs in these individuals are likely to produce discomfort, especially on gaze excursions when the cornea rubs against the top of the plug. Pre-screening the lid configuration may help to avoid this situation. If a plug needs to be removed, forceps can be used to gently raise one edge of the cap, then to grasp the plug by its shaft and lift it straight out (Fig. 9). Trying to remove the plug by the cap itself or by twisting the plug may rip the cap from the body of the plug. Subsequent removal is made much more difficult.

FIG. 9: Jewelers' forceps being used to lift the edge of the cap above the
lid margin.
Silicone canalicular plugs are produced only by Lacrimedics (Herrick plugs). These plugs, which resemble small golf tees, come in two sizes: 0.3mm for small puncta and 0.5mm for larger ones (Fig 10). They are pre-packaged on a fine wire stylus and can be inserted without punctum dilation. These silicone plugs, which have a soft, collapsible bell at the top, are designed to lodge in the restriction at end of the horizontal canaliculus. The tip of the plug is inserted vertically into the punctum until the bell is resting on the punctum (Fig 11). The inserter is rotated until it is parallel with the lid margin and the bell is pushed through the punctum, then 2.0mm to 3.0mm further nasally. The stylus is withdrawn, leaving the plug in the horizontal canaliculus (Fig 12). Tear flow and blink action will cause the plug to migrate 5.0mm to 8.0mm further, until it becomes lodged in place. If necessary, the plug can be removed by irrigation into the naso-lacrimal duct.

FIG. 10: Herrick silicone intracanalicular plug.

FIG. 11: Insertion of a Herrick plug into an undilated punctum.

FIG. 12: Insertion of a Herrick plug into the horizontal canaliculus.
Thermal cautery of the puncta and canaliculi may be indicated if the patient's predisposing condition is permanent. Electro-desiccation using an electrocautery unit also permanently occludes lacrimal drainage. Laser punctal occlusion (punctoplasty) using the argon laser has not been as efficacious as thermal or electric cautery. Surgical repositioning of the punctum anteriorly out of the lacrimal tear meniscus minimizes tear outflow and allows for future surgical adjustments.
Prognosis and Follow-up
In many cases of dry eye, the prognosis is usually guarded, since the treatment may represent only a maintenance strategy. When there is an associated systemic cause, remissions are to be expected when the underlying condition worsens. Multiple evaluations may be necessary to establish the diagnosis and to determine the minimum level of treatment that produces results. Once a treatment plan has shown to be effective, follow-up care at appropriate intervals is necessary to ensure compliance and continued effectiveness.
Reimbursement Tips
Not all insurance plans reimburse equal allowable amounts for the same procedure. Documentation of symptoms and signs as well as accurate coding are required to obtain proper reimbursement from third-party payers. Symptoms must be appropriate for the diagnosis of dry eye. There may be a time interval required between a diagnostic procedure and a treatment procedure.
Medicare has its own specific requirements for requesting reimbursement for a procedure. Use the appropriate ICD-9 codes for diagnoses (Table 3) and CPT codes for procedures (Table 4). Some distributors maintain billing assistance services for their customers.
| DIAGNOSIS | ICD-9 CODE |
| tear film insufficiency | 375.15 |
| keratoconjunctivitis sicca | 370.33 |
| redness or discharge | 379.93 |
| pain in or around the eye | 379.91 |
| PROCEDURE | CPT CODE |
| office visit: established patient | 92012-25* |
| occlude OU lower lids with collagen plugs | 68761-50* |
| occlude OU upper lids with collagen plugs | 68761-50* |
| occlude OU lower lids with silicone plugs | 68761-50* |
| supply of silicone plugs (pair) | 99070(A-4263*) |
| *Medicare uses specific modifiers - check with the carrier before billing | |
Accurate diagnosis, combined with early intervention, will help to maintain the quality of life for individuals with dry eye.
Drs. White and Scott are professors at the New England College of Optometry



