RGP insights
Treatment Trials for Dry Eye and Single-Use Products
BY CHRISTOPHER SNYDER, OD, MS, FAAO
June 1999
Nonpreserved products are recommended for treating more severe dry eye symptoms. The following article will take a look at the available treatment options and other related issues.
General Considerations
Tear supplementation can be helpful, particularly for true aqueous deficient dry eye. Occlusion of the puncta can give an additive effect. These treatments are not as effective for evaporative dry eye, a condition where tear film stability is the problem, not tear volume. An artificial tear is almost always appreciated for temporarily soothing the dry eye, and they are a part of the treatment protocol for any stage of dry eye symptoms. The type of product recommended is based on the severity of symptoms, with nonpreserved artificial tears recommended exclusively for moderate and severe symptoms (Table 1). Preserved artificial tears are avoided because preservatives can destabilize an already compromised tear film. Additionally, the more severe the symptoms, the more frequent the use of the product and the greater potential for cumulative effects of preservatives on the tear film or ocular surface.
| DRY EYE SYMPTOM LEVEL | RECOMMENDED PRODUCT |
| Mild | Preserved or non preserved ATs |
| Moderate | Nonpreserved artificial tears |
| Severe | Nonpreserved artificial tears |
Volumes and Capacities
The normal tear volume on the ocular surface is 8-10�l and a volume of 30�l can be maintained briefly if the eyelids are not squeezed. Excess volume rapidly drains through the normal nasolacrimal ducts.
Packaging Details
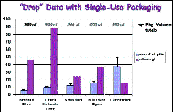
As a function of the packaging design, some of the average drop volumes are so generous that they dispense more than the ocular surface can hold and some drop volumes are much smaller (Figs. 1&2). Note the large variation between products (packaging) and artificial tear "dosage."
|
|
Clinical Messages
The outcome of treatment trials with single-use artificial tear products may be influenced as much or even more by the volume of fluid which is delivered by the packaging as by the unique properties of the fluid. Eyecare practitioners should be aware of this important packaging factor.
Ophthalmic literature cites another packaging issue -- patients who abrade their cornea with the plastic barb that is created by twisting off the endcap of a unit dose artificial tear dispenser. Most current packaging has been designed to avoid this potential problem.
Dr. Snyder is a professor of optometry and serves as chief of contact lens patient care at the School of Optometry at the University of Alabama at Birmingham.