Preventative Contact
Lens Care: Part III
BY RONALD K. WATANABE, OD, AND MARJORIE J. RAH, OD, PHD
AUGUST 2001
This third installment of a three-part series discusses the role
of contact lens solutions in maintaining clean lens surfaces.
Parts I and II of this three-part series on preventative contact lens care discussed the preliminary evaluation and the potential complications associated with contact lens wear. This final installment explores the role of contact lens solutions in maintaining physically and bacteriologically clean lens surfaces that promote healthy and comfortable lens wear. Standards for cleaning and disinfection efficacy are explained, as are regulations and guidelines from the Food and Drug Administration (FDA). Finally, we present a summary of current and upcoming care systems.
Historical Perspective
Contact lens care has changed remarkably in the past few years. From its humble beginnings with daily surfactant cleaners, homemade saline solutions and heating units, lens care has evolved into a highly scientific field of convenient yet efficacious multi-purpose solutions (MPS). Solution manufacturers have been able to develop highly sophisticated formulations that can kill microorganisms, remove lens surface deposits and enhance surface wetting, all without harming the eye. With the options available today, practitioners are able to prescribe a convenient, safe, effective and comfortable solution to all contact lens patients with minimal risk of solution-induced complications. In this convenience-demanding, consumer-driven world of contact lenses, modern contact lens solutions can greatly help patients maintain healthy eyes without having to spend lots of time caring for their lenses.
Role of Solutions
Repeated use of contact lenses results in surface contamination by environmental debris, tear film components and microorganisms. The eye is very efficient and effective at preventing these contaminants from adversely affecting the eye via anti-microbial components in the tears, mechanical flushing by the eyelids and the relatively impenetrable corneal epithelium. However, excessive contamination may contribute to ocular complications such as papillary conjunctivitis, superior limbic keratoconjunctivitis and infiltrative keratitis. In addition, lens comfort and vision may be reduced. Contact lens solutions are designed to remove surface contamination to help maintain healthy eyes. They also aid vision and comfort by cleaning the lens surfaces so that they remain as clear and residue-free as possible. All contact lenses, including disposable lenses, that are worn, removed and reused must undergo cleaning and disinfection between uses. If lenses are not cleaned and disinfected, deposits and microorganisms are more likely to accumulate on the lens surfaces and contribute to ocular complications.
The basic functions of contact lens solutions are cleaning, rinsing, disinfection, rewetting and storage. Many current solutions are able to perform all of these functions, but those with specific purposes are also available. Each solution on the market must undergo specific testing to be approved by the FDA prior to being marketed to the public. Once approved, lens care products must be prescribed and used as indicated by the manufacturer to ensure proper lens cleaning and disinfection. Any deviation from the prescribed regimen may result in reduced efficacy and potential ocular complications.
FDA Approval Process
All lens care products must undergo a specific FDA approval process for Class II devices. To be approved, a product must demonstrate in general a "substantial equivalence" to other similar currently marketed products. Substantial equivalence means that a lens care product has been shown to be as safe and effective as other currently marketed products that have the same usage or function. The FDA may reject a manufacturer's request for market approval if the product is shown to be less safe or efficacious, or if the product has a new intended use.
|
|
|
|
Figure 1. Fungus on soft contact
lens. |
|
In general, with new components or percentages solutions must undergo clinical testing in 60 subjects for 90 days, while solutions with the same components and relative percentages as currently marketed solutions may need only 30 subjects for 30 days (510k procedure). By going through this process, contact lens care products are shown to be safe and effective with a high level of surety for consumers. Each care product undergoes specific testing that includes chemistry, microbiology, toxicology and clinical evaluations. Chemistry investigates uptake and release of preservatives by lens materials, cleaning effectiveness, and solution compatibility to lens materials. Microbiology refers to how effective the solutions are at eliminating specific microorganisms. Toxicology looks at the adverse effects of the solutions on living tissues, specifically the eye. Clinical evaluations are the final step in validating the safety and efficacy of new solutions and care regimens prior to being marketed to the public (Figure 1).
Solution Types
Contact lens solutions can be categorized into saline solutions, cleaners, chemical disinfecting products, MPS and in-eye contact lens solutions (rewetting drops). Saline solutions are basically isotonic salt solutions that are used for rinsing and for storage for heat disinfection systems. Both preserved and unpreserved formulations are available. Saline solutions must undergo testing for microorganism stasis capability prior to marketing. In addition, each bottle is labeled with an expiration date which indicates the shelf life of the solution after manufacturing. Shelf life considers solution stability and microorganism growth. In addition, many solutions have recommendations for when a solution bottle should be discarded after opening. These are dependent upon the preservatives in the formulation, storage temperature and environment in which the bottle is used.
Rewetting drops are essentially saline solutions packaged in smaller bottles. Some rewetting drops may contain additional lubricants, wetting agents and cleaning components to enhance the performance of the solutions. For example, Alcon's Clerz Plus contains two surfactants (Clens-100 and Tetronic) to help loosen and remove surface debris during lens wear. Allergan's Refresh Contacts provides lasting mucomimetic action due to hydroxyproply methylcellulose (HPMC) and cytoprotection from carboxymethylcellulose (CMC). Rewetting drops must be packaged in bottles no larger than 30 ml because of the risk of contamination. Instruct patients on the proper use of rewetting drops to prevent solution contamination.
Disinfecting Products
Solutions for contact lens disinfection are categorized as Chemical Disinfecting Solutions, Chemical Disinfection Systems, or Conditioning Solutions. Though the goal of eliminating microorganisms is the same for all of these categories of solutions, there are some differences in how they are approved for use.
A Chemical Disinfecting Solution is a single solution that contains preservatives or other anti-microbial agents in sufficient concentration to destroy bacteria and other harmful microorganisms by itself. To be approved, the solution must meet specific minimum standards for stand-alone reduction of microorganisms (Table I). This means that simply storing lenses in the solution will reduce each bacterial species 1,000 times and each fungus 10 times. This claim does not require separate rubbing and rinsing steps. Some contact lens solutions labeled as MPS may not meet the stand-alone disinfecting criteria and would therefore not be considered a disinfecting solution.
|
TABLE 1: Stand-alone Disinfection Efficacy Criteria |
|
| TEST ORGANISM | REDUCTION OF INOCULUM OF ORGANISMS |
| Pseudomonas aeruginosa |
|
| Staphylococcus aureus | |
| Serratia marcescens | |
| Candida albicans |
|
| Fusarium solari | |
A Chemical Disinfection System is a solution or care regimen that consists of separate rubbing, rinsing and storage steps. This can be accomplished by a single MPS or a care system with multiple solutions. To be labeled as a chemical disinfection system, the regimen must be able to reduce the total bacterial count by at least five log units (at least 1.0 log unit for any given organism) and be able to hold fungi to stasis levels throughout the recommended disinfection period, which can range from five minutes to six hours, depending on the system. In addition, laboratory testing procedures must demonstrate that inoculated lenses that have undergone the steps outlined in the care regimen must not culture out more than 10 colonies of any organism. These criteria ensure that although the lenses are not sterilized, they are disinfected to safe levels when the care system is used properly. Misuse of the system through modification or omission of steps may reduce the effectiveness of the care regimen. Most MPS are approved as disinfection systems and include rubbing and rinsing steps in addition to minimum storage times. Hydrogen peroxide systems also require a neutralization step prior to lens wear.
Conditioning Solutions disinfect and enhance the wettability of rigid gas permeable (RGP) lenses. Because RGP lenses are relatively hydrophobic, special wetting agents are added to create a thin hydrophilic layer on the lens surface that makes the lens more comfortable upon initial application. In addition, the tear film is better able to form a stable layer on the lens surface, and dry spots are reduced with overnight soaking. Conditioning solutions generally meet the anti-microbial requirements of disinfection systems and must be accompanied by digital rubbing and/or a separate daily cleaner.
Multi-Purpose Solutions
By far the most popular type of solution today is the MPS. The MPS has been approved as a single-bottle system for cleaning and disinfection of contact lenses, but separate cleaners for surfactant cleaning, abrasive cleaning and enzyme removal may be added by the practitioner. Depending on the specific solution, the cleaning and disinfection steps may include digital rubbing, rinsing and storage. One current goal for MPS manufacturers is to reduce the number of steps (i.e., digital rubbing) required to achieve acceptable levels of cleaning and disinfection. MPS solutions are able to achieve adequate cleaning and disinfection through complex formulations of preservatives, surfactants, lubricants, buffers and ionic agents. Each component is an integral part of making sure the lens is safe to wear the next day. It is important to distinguish between MPS products that have stand-alone approval vs. those that require digital rubbing as part of the disinfection regimen. In addition, it is important to read the manufacturers' instructions for products that do not require digital rubbing because a critical rinsing step is required. Patients who are not instructed to use the solutions properly may find decreased lens performance over time.
Solution Components
Preservatives Preservatives are compounds that reduce the bacterial and fungal loads placed on contact lenses during normal use. Most modern preservatives are large molecular weight derivatives of older preservatives and tend not to be absorbed into soft lens matrices or bind to lens surfaces as readily as older preservatives. Common preservatives include polyaminopropyl biguanide (Dymed, a chlorhexidine derivative), polyhexamethylene biguanide (Tris-Chem, a chlorhexidine derivative), polyhexanide hydrochloride (PHMB, a chlorhexidine derivative), polyquaternium-1 (Polyquad, a quaternary ammonium) and myristamidopropyl dimethylamine (Aldox). These preservatives are effective against common bacteria and fungi when the lens is stored in the solution for the minimum recommended soak times. They are also effective for longer storage times of up to 30 days, depending on the solution. Most of these preservatives are not effective against Acanthamoeba cysts and trophs. Myristamidopropyl dimethylamine may be the one exception that is effective against protozoa. For patients in environments with a high risk of Acanthamoeba contamination, this may be the best preservative to use.
|
|
|
|
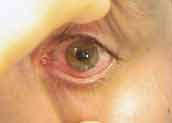
Figure 2. Red eye reaction due to contact lens solution preservative
sensitivity. |
Preservatives have also been known to induce adverse tissue reactions. First-generation preservatives such as thimerosal, chlorhexidine and benzalkonium chloride routinely created red eye reactions in soft contact lens wearers (Figure 2). Both toxic and allergic reactions to these molecules were common, especially as the preservatives increased in concentration within the lens matrices. Because the newer preservative molecules are larger and tend not to penetrate the lens matrices, these types of reactions have largely been eliminated. However, sensitive patients or those who demonstrate poor lens care habits still develop allergic or toxic responses to preservatives. These patients may then require preservative-free care systems to prevent recurrence of the sensitivity reactions.
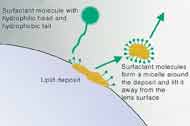
Surfactants Surfactants are chemicals that reduce the surface tension of solutions to facilitate debris removal from lens surfaces, usually in conjunction with digital rubbing. In essence, the amphipathic nature of surfactants (hydrophilic head and hydrophobic tail) utilizes ionic charges to displace deposits from the lens surface (Figure 3). When used properly, surfactants help remove loosely bound oils, mucous and debris. Common surfactants include Poloxamine, Poloxamer, Pluronic and Tetronic.
|
|
|
|
Figure 3. Action of surfactants on hydrophobic surface deposits. |
|
Most MPS formulations contain a surfactant to facilitate the removal of loose surface debris. Though the surfactants are present at low levels, sensitive patients may notice slight stinging or lens dryness due to the action of the surfactant cleaners. Patients who deposit heavily or wear conventional contact lenses should be advised to use a separate daily cleaner with surfactants to maintain cleaner lens surfaces.
Wetting Agents and Lubricants Comfortable lens wear relies on lens surfaces that maintain good wettability. Poorly wetting surfaces create friction between the lens and the cornea and eyelids, which may lead to irritation and adverse responses. Many solutions, particularly rewetting drops and RGP conditioning solutions, contain wetting agents, lubricants and/or viscosity agents to enhance lens wettability. Common wetting agents and lubricants include povidone, hydroxypropyl methylcellulose, polyethylene glycol (PEG) and dextran.
Protein Removers Denatured protein can become problematic for contact lens wearers, particularly those who wear conventional lenses. Both soft and rigid lenses can accumulate protein over time, and once the protein has reached a certain level, the wearer will start to notice decreased comfort, wearing time and vision. The practitioner may notice ocular changes such as corneal staining and papillary hypertrophy on the upper tarsus. Traditionally, enzymatic cleaners have removed protein. Enzymatic cleaners such as papain, pancreatin and subtilisin are available in both tablet and liquid forms and are generally used on a periodic basis. Regular use helps prevent excessive protein build-up on lenses, though eventually even the most diligent patient will have to replace his or her lenses due to deposit accumulation (Figure 4).
|
|
|
|
Figure 4. Protein deposits on the posterior periphery of an RGP
lens. |
Patients using current MPS and hydrogen peroxide systems have enjoyed the use of enzymatic cleaning tablets that are dissolved in the overnight soaking solution, a feature that has simplified this step for many lens wearers. Liquid enzymatic cleaners, such as Alcon Supraclens and Boston Liquid Enzymatic Cleaner, have also helped increase patient compliance with protein removal. Instead of dissolving an enzyme tablet in the soaking solution, the patient can simply place a drop or two of the liquid enzyme in the soaking solution. Overnight soaking followed by a rinse before lens application completes the cycle. With this added ease and cost reduction per cycle, enzymatic cleaning can be recommended as often as daily.
Current MPS products are also claiming protein removal via non-enzymatic processes. Because enzymes are irritating and potentially harmful to the eye, they have not been included directly in MPS formulations. However, other protein removers have been added to some solutions.
|
|
|
|
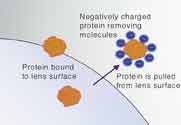
Figure 5. Protein removers such as Hydranate and Citrate use ionic charges to pull protein molecules from the lens surface. |
|
Hydroxyalkyl phosphonate (Hydranate) in Bausch & Lomb ReNu MultiPlus solution is a chelating agent that ionically pulls protein molecules off of the lens surface. Sodium citrate, the buffer in Alcon Opti-Free Express, is a negatively-charged molecule that pulls positively-charged protein molecules from the lens surface using its surface charge (Figure 5). As newer formulations are developed, we will continue to see new protein removing components in MPS products.
Care Systems
As described earlier, most care products for soft and rigid lenses are prescribed as a system that cleans and disinfects the lenses. Care systems can range from single-bottle systems to more complex and specialized multiple solution systems. Determining specific characteristics will help determine the most appropriate care system for a patient.
Single-Bottle Systems Single-bottle MPS systems are the most convenient and popular of the care systems types. By including cleaning, rinsing, disinfecting and storage capabilities into one solution, the patient has very little to think about. New formulations simplify the system further by eliminating certain steps, such as digital rubbing and enzymatic cleaning. Currently, Alcon Opti-Free Express, Allergan Complete and recently-launched CIBA Vision AO Sept Clear Care have a no-rub indication, with Bausch & Lomb soon to follow (Figures 6 and 7). Because many patients admittedly skip the rubbing step, especially when wearing disposable lenses, using a solution that does not require the rubbing step is of great benefit. Whereas reduced cleaning and disinfection efficacy without the rubbing step was a previous concern, these solutions reduce that concern. However, it is still important for patients to follow the manufacturer's instructions for solution usage. Opti-Free Express requires a five-second rinse on each lens surface before and after soaking to attain maximum cleaning and disinfection. Patients who are not instructed to perform this step may suffer from poor cleaning despite the no-rub label.

|

|
| Figure 6. Opti-Free Express and ReNu MultiPlus solutions feature protein removal in a multi-purpose solution. | Figure 7. Complete and SoloCare multi-purpose solutions provide single-bottle disinfection for soft lens wearers. |
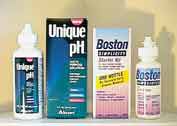
RGP lens care has headed towards the MPS as well. Currently, Boston Simplicity and Alcon Unique pH provide RGP wearers the same convenience that soft lens wearers enjoy years (Figure 8). Simplicity combines the preservatives chlorhexidine and Dymed with a betaine surfactant, PEO sorbitan monolaurate (cleaner), a cellulosic viscosifier and devitalized PEG as a wetting agent. Unique pH contains PEG as a lubricant, Tetronic 1304 as a surfactant and Polyquad as the preservative. It also contains a proprietary hydroxypropyl guar/borate polymer system that changes the viscosity of the solution with pH. The proprietary polymer system keeps the solution at low viscosity while in the bottle and during cleaning and rinsing, but it increases viscosity once in contact with the higher pH ocular surface. The result is a solution that is easy to work with during lens handling but one that provides cushioning and wetting when the lens is placed on the eye. Both Simplicity and Unique pH are comfortable yet effective solutions for rigid gas permeable lens wearers, but patients who skip the rubbing step or are heavy depositors may find rapid deposit build-up and decreased lens performance.
Hydrogen Peroxide Care Systems Despite the popularity of MPS systems, hydrogen peroxide (H2O2) systems have maintained their popularity among sensitive eyes patients. Peroxide has the distinct advantage of being preservative-free, which effectively eliminates the adverse effects associated with preservatives, as long as the H2O2 is neutralized. It is therefore a good choice for patients with allergies. In addition, peroxide systems have demonstrated good cleaning and disinfection efficacy over the years, which makes them a great choice for those patients wearing conventional soft lenses.
|
|
|
|
Figure 8. Unique pH and Simplicity are multi-purpose solutions for rigid gas permeable contact
lenses. |
Despite their effectiveness, peroxide systems have been plagued with the reputation of being complicated and expensive. Current systems, such as CIBA's AOSept and Allergan's UltraCare, require separate cleaners, a neutralizer and enzyme tablets. CIBA made things a little simpler with Pure Eyes, which uses an MPS for cleaning and rinsing, but a separate peroxide soaking solution is still required. Recently CIBA introduced AOSept Clear Care, a no-rub, preservative-free peroxide system. It will still use 3.0% H2O2 to disinfect, but the formulation will include a Pluronic surfactant to add cleaning efficacy. This solution will merge the simplicity of MPSs with the qualities of peroxide.
One final system that is not truly a peroxide system is CIBA's QuickCare. This system has a cleaning/disinfecting solution that is based on its MiraFlow Extra Strength Cleaner, which contains 20 percent isopropyl alcohol. The cleaner disinfects during the digital rubbing step, and requires 10 drops of solution and 30 seconds of rubbing. The rinsing/storage solution is SoftWear saline, which contains perborate, which releases low levels of peroxide and acts as a preservative. The low levels of peroxide are easily neutralized by the preocular tear film.
RGP Care Systems
RGP lens care systems have until recently been exclusively two-bottle systems containing separate cleaning and soaking solutions. Boston Original and Advance Comfort Formula systems both utilize an abrasive daily cleaner and a conditioning solution that disinfects and wets lens surfaces. Alcon OptiSoak uses a similar set-up.
Lobob Optimum is a slightly different system that utilizes a cleaning/disinfecting/soaking solution containing benzyl alcohol, a separate extra-strength cleaner and a wetting solution. It is different because the soaking solution cannot be used in the eye, so the lens must be rinsed and rewet with the wetting solution prior to application in the morning. Because it is non-abrasive, it is a good option for softer RGP materials, and its alcohol content makes it effective against lipid deposits.
One final RGP solution that also doubles as a soft lens solution is CIBA's SoloCare. It is the only cleaning and soaking solution that can be used for both soft and rigid lenses. It contains polyhexanide HCl as its preservative and has a 10-minute disinfection time. Its only drawback for RGP wearers is its low viscosity, but for patients who are using SoftPerm lenses or piggyback systems, it provides maximum convenience.
Summary
The importance of contact lens solutions is often overlooked or diminished by practitioners and patients. However, they are the primary means by which patients can maintain clean lenses and good ocular health during contact lens wear. Using good judgment in prescribing care systems and providing thorough patient education enhances the patient's vision and comfort while minimizing ocular complications. Finally, non-blister pack diagnostic lenses must be disinfected properly, preferably with heat, to minimize the chances of spreading infectious diseases. A strong working knowledge of contact lens solutions goes a long way in ensuring the success of your contact lens patients and practice.