DRY EYE TREATMENT
Potential Treatments for Dry Eye
By Donald L.
MacKeen, MS, PhD
February 2001
This first of three articles on treating dry eye examines U.S. patents of the past decade for eye drops, sprays and punctal occlusion.
In the past few decades, interest and research into dry eye, keratoconjunctivitis sicca (KCS), has increased enormously, giving rise to large numbers of United States patents and research publications. Improved treatments are not reaching patients with the same frequency. A dry eye subject survey by Kozma et al. (2000)* reported that more than 76 percent of patients rated their conditions as the same or worse despite treatment compared with the previous year. Even though treatment efficacy has not kept pace with the publication of patents, patenting a treatment increases the probability that it will become available to the patient.
This is the first of three articles covering all aspects of topical dry eye treatment, based primarily on United States patents awarded in the last decade. This part covers conventional eye drops, sprays and punctal occlusion. The second will address replacement of natural tear components or hormones and the final part will cover topical pharmacological treatments.
Punctal Occlusion
The punctal openings near the nasal canthi enable fluid to flow out through the canaliculi. One can insert hand-formed gelatin plugs or short sections of collagen suture material to temporarily block the puncta. Gokhale (1998) advocated applying cyanoacrylate to occlude the punctal opening. These openings may be permanently closed by cautery to preserve tears in severe dry eyes. Initially done with an electric cautery needle, it has also been done with laser radiation. Hutnick et al (1998) compared the efficacy of thermal and Argon laser cautery at one and six months and found thermocautery more effective.
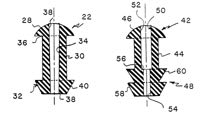
Silicone plugs provide longer lasting, reversible occlusion. The original silicone plugs were held in place by the ring of tissue subjacent to the punctal opening. Problems with these devices, including insertion, loss, discomfort and irritation to the globe, resulted in several variations in the last decade. MacKeen and Roth [1990] designed a silicone plug with a patent central canal to enable some outflow (Figure 1). The open flow punctum plug could prevent tear stagnation. Mendius [2000] patented a plug with variations on the protruding portion that reportedly lessens globe irritation. Other variations include Mendius' [1998] accordion-shaped or Webb's [2000] screw-shaped model intended to be threaded into the canaliculus (Figure 2).
|
|
|
Herrick et al [1991] developed another variety of occlusion that utilizes canalicular implants. Herrick's [1992] device is inserted into the horizontal canaliculus. Seder et al [1990] described his invention as a reversible, flexible device formed of a shaft having a low profile cap at one end and a rounded tip at the other end with up to three conical flanges on the shaft. The rounded tip enters the punctum followed by leading edge of the range(s) until the occluder bends and enters the horizontal canalicular canal. Rumelt et al (1999) reported that canaliculitis and dacryocystitis resulted from migrated silicone punctum plugs. Although simpler to insert, early versions were reportedly prone to migrate through the canaliculus into the nasopharyngeal area.
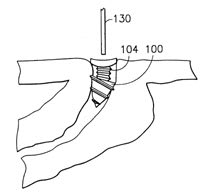
Two patents were filed for in situ plugs. Schmitt [1995] patented a system comprised of main or side chain crystallizable polymers (Figure 3). A thermoplastic gel is liquefied with gentle warming, then injected into the punctal opening where it gels on contact with the lid tissue. One can remove the plug if desired by gently warming the adjacent lid area. Hamano [1998] patented a solution formulation intended for injection into the canaliculus. The liquid consists of gelatin, agarose, a mixture of fibrinogen and thrombin, a polysaccharide and a calcium salt. Two liquid components are mixed during punctal injection, and a gel plug forms on contact with the warmer lid tissues (Figure 4). Both the Schmitt and the Hamano plugs conform to the unique contours of the caniculi; reportedly no material protrudes from the punctal opening, and the plugs do not migrate.
|
|
Figure 4. Two solutions mix to form an in situ gel plug. |
Artificial Tears
Artificial tears have been used for centuries to treat dry eyes. This form of treatment presents difficulties in patient instillation and short duration of benefits. Lemp stated in 1973 that, "...instilled solutes are diluted...to 1/1000 of their original concentration within eight minutes." Patients are also often troubled by spillage of instilled tears, which can ruin carefully applied mascara.
Artificial tear formulations have become increasingly complex over the years in attempts to increase comfort and acceptable contact with exposed ocular tissues, and to prolong their duration.
Instillation Difficulty
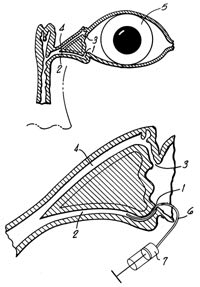
Py [1990] states in his patent: "Frequently the elderly are the recipients of ocular treatment. In general, the elderly may have poor near vision and may also suffer from tremors and finger arthritis. For this reason, when liquid medicament must be applied to the eyes of an elderly individual, it generally must be applied by a third party in 70 percent of all patients according to Kass." Py's device intends to facilitate self-administration and consists of a complex apparatus attached to a spectacle frame (Figure 5). When a patient faces a mirror and rotates his/her eyes upwards, the prism shows the digitally everted lower cul de sac. Medication contained in the reservoir is delivered to the conjunctiva. The device seems rather bulky and presents difficulties in maintaining sterility in the reservoir.
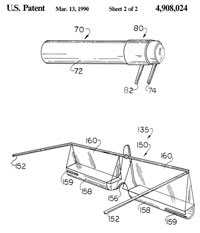
Figure 5. Artificial tear self-administration
device
Vo [1992] patented a delivery system comprising a fluid reservoir for holding a supply of eyedrop fluid, tubing to conduct the fluid to the eye and fluid control means to drive the eyedrops from the reservoir through the tubing to the eye.
Basilice [1994] patented an eyedrop dispensing device that fits on the dispenser of an eyedrop squeeze bottle and positions against a patient's lower eyelid. The assembly can be rotated to bring a nozzle tip to face the eye. A protrusion of the dropper cap presses against and everts the lower eyelid. Lifting the dropper bottle brings the nozzle over the everted cul de sac for instillation.
Spray Devices
Spray devices attempt to foil reflex blinking during drop instillation. An effective spray can be directed at the interpalpebral spaces where dry eye treatment is needed, its volume is small and should not overwhelm the normal resident volume, and it could be engineered to nearly mimic the lacrimal glands.
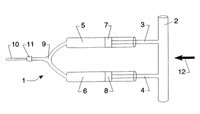
Spray devices are usually attached to spectacle frames. Bertera [1994] patented a spectacle-like device that can project droplets of liquid onto the wearer's eye. It features a medication reservoir, a rotating wiper that acts as a valve on the tubing, a pump-controlling electrical circuit and a spring to provide continuous pressure on the reservoir (Figure 6) This device requires batteries, adding to previously noted problems with frame-attached devices with reservoirs.
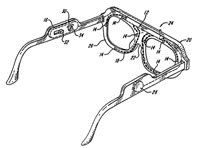
Figure 6. Spectacle-like spray device
Bertera [1998] described a spray device that could eliminate some of these disadvantages by utilizeing ink-jet technology developed for computer printers. The patent abstract states: "a spectacle-like device mounts a system of pump elements that can project droplets of liquid onto a wearer's eye. A reservoir of the liquid and an electrical pump-controlling circuit are mounted on the spectacle frame for operation with the pump elements."
Contact Time of Instilled Drops
Many have attempted to increase the contact or dwell time of aqueous solutions, primarily by increasing the viscosity of the instilled material. There are three viscosity categories: 1) Newtonian solutions, in which the viscosity is independent of lid movement, 2) non-Newtonian (thixotropic) in which the viscosity is greatly lessened during lid movement and 3) gelling solutions that are transformed from liquid to gel on instillation.
When viscosity increases, discomfort increases. Adler et al reported that ophthalmic solutions greater than 15 cp generally have decreased comfort. Paugh et al found the residence time of CMC solutions was approximately 10 minutes for viscosities that were comfortable and 20 minutes for those that were excessively viscous. This is an increase of over four minutes as compared to instilled saline.
Newtonian Solutions. Newtonian solutions exhibit viscosity that is independent of the shear rate. Increased viscosity intended to increase contact time causes lid drag and patient discomfort. Conti [1989] patented dry eye solutions containing 0.1% to 0.5% of a methylated chitosan selected from the group consisting of N-dimethylchitosan and O-methyl-N-dimethyl-chitosan. Chitosans are known for their coating and lubricating qualities. Such solutions reportedly have improved residence time.
Lang [1995] described an alkaline pH solution containing carrageenan that creates a "non-irritating irritating emulsion for sensitive eyes." The alkaline pH may make this solution somewhat similar in pH to the British product Hypromellose.
Manzuel [1991] patented the use of Rhamsam gum for such formulations. Unlike most natural products used for this purpose, it can withstand sterilizer temperatures.
Non-Newtonian Solutions. Non-Newtonian or thixotro-pic solutions are gels which become liquid and flow when put in motion by blinks or ocular movements. A gel coats the intrapalpebral area between blinks. A proper formulation assures that the gel layer persists but is thin enough to avoid blurring.
Dikstein [1993] patented solutions with non-Newtonian rheological properties that have a low salt content and adjust to achieve an acceptable viscosity during the blink. The solutions comprise anionic polymers (hyaluronic acid, polyacrylic acids or carbomers) plus a polyol such as glycerol.
Bron et al (1998) reported on a clinical study which compared a solution containing 0.2% polyacrylic acid (PAA) (a non-Newtonian solution) with 1.4% Polyvinyl alcohol (PVA) in 89 dry eye patients. PAA patients had improved signs and symptoms and lower frequency of instillations, but some reported blurred vision.
Gressel et al [1991] patented a carboxy vinyl polymer gel for dry eye syndrome consisting of polyanionic polymers and a carbomer that possesses mucomimetic, rheological and lubricating properties, with a polyol stabilizer. Reportedly the gel "flows" during the blink. Their aim was to create a treatment that would last from four to 24 hours. The patent provided no experimental or clinical data regarding enhanced contact times.
Pena et al [2000] patented an aqueous preparation of aloe vera extract and HEC that forms a thixotropic ointment when Aquaphor is added. Reportedly it assists in healing. Pena suggests applying the ointment form using the supracutaneous lower lid application method first described by MacKeen (Part II of this series). The authors report benefits in treating Sjögren's syndrome dye eyes.
Gelling Solutions. Solutions that become gels should coat exposed ocular surfaces, but the thickness should not decrease visual acuity. Changes in pH, ionic strength or temperature can initiate the transformation from liquid to gel. Gelling preparations are either irreversible or reversible. Irreversible, thermally-actuated gels are difficult to ship and store.
Hoeg [1995] describes a reversible aqueous and oil emulsion that gels in response to changes in temperature and pH. Kabra [1998] describes emulsions comprised of a nonionic cellulose ether, oil, water and optionally an emulsifying agent that changes from a liquid to a gel following instillation because of the increased temperature of ocular tissues.
Plant extracts such as agar, xanthine gum, carob and locust bean gum have been utilized to enhance the viscosity of eye drops. Many of these formulations change from liquid to a gel after instillation based on change in pH or tonicity. Most are unable to withstand the high temperatures for sterilization.
Missel et al [1993] patented a gelling polysaccharide of carrageenans, xanthine gum, locust bean gum and gellan gum. Although designed as a drug carrier rather than a dry eye treatment, this reversibly-gelling formulation based on a change in ionic strength or pH can also be formulated as thixotropic.
Joshi [1993] patented reversibly gelling aqueous compositions that gel following simultaneous changes in both temperature and pH. The solutions employ dicarboxylic acid, methocel, Carbapol, Pluronic RTM/Carbopol and Tectronic. The formulation, a liquid with an acid pH at room temperature, becomes a gel at body temperature and pH 7.4.
Saettone et al [2000] patented a solution containing tamarind seed polysaccharides reported to possess both pseudoplastic and mucomimetic qualities that may improve contact time in the treatment of dry eye conditions.
Decrease Evaporation
One can use spectacles (with or without side panels) or swimming goggles to ameliorate moderate to severe dry eye caused by evaporative loss. Swimming goggle wear may be cosmetically unacceptable and is usually reserved for severe cases.
Scheiner [1997] patented a device consisting of a reservoir chamber attached to spectacle frame that closely fits over one or both eyeballs and the adjacent cheeks. It is grossly similar to goggles, but has an aqueous reservoir connected to release chambers containing absorbent material, which is assumed to provide elevated humidity for the dry eye (Figure 7). The feasibility of this device remains to be seen. The patent presented no data regarding its efficacy. As with similar devices, one common problem is maintaining solution sterility.
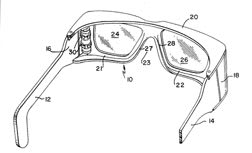
Figure 7. Goggle-like device to prevent tear evaporation.
To receive references via fax, call (800) 239-4684 and request document #68. (Have a fax number ready.)

*Note: Dates of patents are in brackets [ ] and dates of scientific papers are in parentheses ( ).