DRY EYE TREATMENTS
Potential Treatments for Dry Eye, Part II
By Donald L.
MacKeen, MS, PhD
March 2001
This second of a three-part series examines U.S. patents for treating dry eye by adding tear and lacrimal gland components.
This is Part II of a three-part series of articles which discuss U.S. patents and research of the last decade generated by interest in dry eye treatment. This article covers patents and research related to adding tear components, such as lipids, and lacrimal gland components including electrolytes, lipocalin, androgens and estrogens.
Part III will focus on topical pharmacological treatments.
Lipids
The pre-ocular tear film lipid layer in part retards evaporative loss from the subjacent aqueous layer. Early attempts to enhance the lipid layer utilized direct application of ointments or instillation of light liquid petrolatum, but these overly thickened lipid films decreased visual acuity for a substantial period of time.
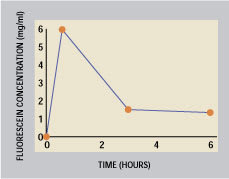
Figure 1. Duration of a fluorescein marker
from supracutaneous delivery (From Tsubota
et al. British Journal of Ophthalmology
Vol: 83(7) p. 769 (1999))
In a series of patents, MacKeen [1994a&b, 1997, 1998] describes a lipid formulation applied to the skin of the lower eyelids (supracutaneous delivery). This method supplies lipids to the inferior meniscus over an adequate period of time without decreasing visual acuity. The protracted delivery time is shown in Figure 1 (Tsubota et al 2000). The patented calcium ointment (DEO) is streaked with a fingertip or a cotton-tipped applicator over the lower lid skin (below the lashes) from 6 o'clock to the lateral canthus. Figure 2 shows the placement site streaked with zinc oxide (used in place of undetectable DEO ointment for photographic purposes). Previously unreported connections between the orbicularis and the lid skin move lipids on this site towards and over the lid margin. The lipid and the calcium carbonate enter and mix in the meibomian lipid layer, then spread over the interpalpebral space. A single 30 mg application can relieve discomfort and staining for an entire day, according to MacKeen et al (1998 a, b), Roth (1998), and Tsudoda et al (1999).
Glonek et al [1994] patented a meta-stable emulsion of phospholipids that contains a surfactant and provides a measured volume of lipids. This formulation reportedly provides some natural meibomian lipid components to enhance the aqueous portion of the preocular tear film. Meibomian components within an aqueous vehicle could provide needed water and enhance the critical lipid layer and/or its interface.
Egerer et al's [1995] patent describes the addition of an aliphatic dicarboxylic acid (sebacic acid as sodium or calcium salts). The formulation tested clinically also included methylcellulose. It reportedly decreases the evaporative rate by increasing the oil layer. They claim that 59 out of 60 patients liked the solution, and approximately 50 percent of the patients in the study elected to use the solution over a period of two years.
Guo [1986] describes an aqueous liposome suspension. The lipid composition comprises about 70 to 85 mole percent hydrogenated phosphatidylcholine and 15 to 30 mole percent benzyldimethylstearylammonium chloride with cellulose ethers, PVA or PVP. Reportedly it is stable at room temperature for "several months." The liposomes assumedly bind to the ocular surface. However, the authors did not discuss penetration of the overlying mucus barrier. They did note that liposomes undergo oxidation/peroxidation and hydrolysis, and didn't retain clarity even when refrigerated. Some rabbit data suggests persistence of instilled material on the eye.
Ding et al [1999] described an emulsion of higher fatty acid glycerides, polysorbate 80 and an emulsion-stabilizing agent of Permulin in water, Carbomer 1342 with or without castor oil and glycerin. They reported that this formulation formed a lipid monolayer on the tear film that remained detectable three hours following instillation. There were no data regarding the fate of the aqueous portion.
Blepharitis can cause excessive lipids in the tear film. Extemporaneously-prepared solutions of acetyl cysteine can remove abnormal lipids. A recent patent by Smith [2000] describes formulae consisting of non-ionic surfactants for removing problematic lipids. This may be an alternative in treating blepharitis.
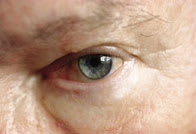
Figure 2. The white layer demonstrates
the placement site for supracutaneous
delivery to the tear film.
Mechanical Inserts and Sprays
Ocuserts appeared in the '70s. These laminated oval inserts were placed in the lower cul de sac (fornix) to provide continuous delivery of drugs such as pilocarpine. This was prior to substances such as lacrimators that might be released in the region of the accessory lacrimal glands.
Kaufman [1990] patented an artificial tear suspension system, including bioerodible mucin-type particles, lipophilic and hydrophilic materials. The mucin-type particles are suspended in either the lipid-type material, the aqueous-type material or both. Once instilled, the product molds to the anatomy of the fornix (Figure 3). The system provides needed components of the natural tear film layers.
Domb [1997] recently patented another drug-delivery insert. He described a biologically inert non-allergenic insoluble insert to which drugs reversibly bind either covalently through hydrolyzable anhydride or degradable methylene diester bond linkages. It could possibly deliver some pharmacological agent, such as stimulants, to functional accessory lacrimal glands or goblet cells.
Kang [1997] patented a water in oil emulsion to be nebulized or instilled onto the ocular surface. The aqueous phase could contain agents from cellulose ethers, vitamins, pilocarpine, etc. He provided no experimental or clinical data.
Replacement Therapy
Many components comprise the tear film, including biopolymers, hormones, vitamins and salts. A dysfunctional lacrimal gland can cause a decreased concentration of any or all of these materials vital to the physiology of the anterior segment epithelium, which in turn is necessary for a stable tear film.
Estrogens, androgens, meibomian fluid components, growth factor, cyclic nucleosides, hyaluronic acid derivatives, ionic calcium and mucin have been investigated or considered for replacement. Current research has identified the various biological and inorganic agents necessary to a stable pre-ocular tear film. When one or more major missing components can be identified, appropriate replacement therapy might help treat dry eye. However, such therapy has foreseeable problems: availability, cost, stability and appropriate delivery to needed sites.
Replacement Lacrimal Gland Fluid?
Pflugfelder et al (1997) have cultured human lacrimal gland acinar epithelia which secrete proteins typically produced by the lacrimal secretory acini in vivo. They have shown it produces TSP (tear specific prealbumin, or lipocalin), lactoferrin, as well as TGF beta and factors capable of binding it. These cultures can be used in screening drugs that stimulate or inhibit secretory function in addition to treating lacrimal gland dysfunction. Such a generator of lacrimal fluid could possibly become part of a short intensive therapy for certain problem dry eyes.
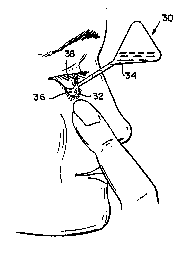
Figure 3. A basic method for
the delivery of tear components in the
initial liquid state. The material will form
a semi-solid capable of releasing tear
components. Kaufman [1990]
Electrolytes
Following his earlier patent of an electrolyte dry eye formulation (TheraTears), Gilbard [1990] patented a range of electrolyte ratios. These included chloride salts of sodium, potassium, calcium and magnesium, sodium phosphate and bicarbonate. The solutions were deemed non-toxic and could be used for dry eye as well as for irrigation of the ocular surface, drug delivery or contact lens maintenance. He assessed the lack of toxicity by the percentage of rabbit conjunctival goblet cells remaining after 12 hours of in vivo immersion. When compared with five known electrolyte formulations (Unisol, lactated Ringers, etc.), he demonstrated the superiority of all his formulations. Gilbard (1992) reported that his electrolyte solution significantly improved goblet cell density, corneal glycogen and tear osmolarity compared with three other dry eye formulations in the treatment of KCS.
Bicarbonate. Edelhauser et al (1998) noted that bicarbonate is derived from the lacrimal gland and conjunctiva. Normal tight junctions (TJ) allow the necessary epithelial surface layer of mucin. These authors also noted that bicarbonate is necessary for maintaining the interface of the PTF and the epithelium. One new artificial tear, Bion Tears, capitalizes on this. Lopez et al showed that buffering with bicarbonate can restore damaged epithelium.
Beck et al [1995] patented a method of stabilizing bicarbonate by adding carbon dioxide under pressure to bicarbonate solution until equilibrium is reached.
The carbonate moiety of calcium carbonate (MacKeen [op.cit]) becomes bicarbonate in aqueous tears.
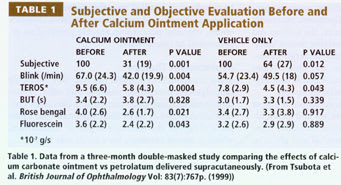
Calcium. This ion is essential in anterior segment physiology for mucin viscosity and TJ. Bion Tears contains both calcium and bicarbonate. Tsubota et al (1999) show the clinical effects of supracutaneously-applied calcium ointment vs the vehicle twice daily by dry eye patients for three months (Table 1). This treatment is currently under IND clinical investigations.
Zinc. Prior to the 1950s, zinc sulfate was the major component of extemporaneously-prepared eye drops prescribed. Saatei et al (1991) reported that the concentration of zinc in normal tears is greater than in serum. Shankar and Prasard (1998) reported on zinc's role in the immune system, and its anti-oxidant action. Zinc chloride is present in Bion Tears and Dry Eye Therapy artificial tears.
Vitamins. Dry eye from avitaminosis A garnered much interest and clinical activity during the '80s because all signs and symptoms disappeared on oral administration of vitamin A. Many clinical studies investigated effects of topical vitamin A in dry-eyed subjects, but the results were not dramatic, and topical use of retinol cogeners or items with vitamin A activity is not currently popular.
Itoh et al [1999] patented vitamin D for topical use primarily to treat corneal haze after corneal damage but also to treat dry eye.
Hyaluronic acid. Berry et al (1998) found the glycoprotein hyaluronic acid (HA) in measurable concentrations in most normal tears. Chemically pure HA is readily available. Adding HA 0.05% to 2% to an artificial tear may enhance residence time of instilled tears. Solomon and Merin (1998) described the beneficial effects of drops containing HA and glycerol compared with conventional eyedrops. Cantoro [1998] patented such a formulation also containing bicarbonate in a hypotonic solution. He believed increased viscosity produced the beneficial action of HA, but other researchers have differing opinions. Avisar et al (1997) reported that the benefit of eyedrops containing HA was independent of the viscosity; the 0.1% solution had the best influence on the PTF. It is possible that formulations containing this substance benefit the dry eye both by replacing an element ordinarily present in lacrimal gland fluid and enhancing residence time.
Sex Hormones
Androgens. Androgens like TGF beta have been advocated for dry-eyed women, especially those with Sjögren's syndrome. Mircheff (1996) and Sullivan et al (1998) reported the decreased androgen tear concentration and/or decreased androgen receptors in postmenopausal women and Sjögren's syndrome sufferers may decrease meibomian gland activity. Androgen treatment seems appropriate for postmenopausal patients whose meibomian and lacrimal gland tissues possess adequate receptors to respond to the treatment. However, its stimulating effects on meibomian glands might initiate a recurrence of excessive abnormal meibomian secretions in previous meibominitis sufferers.
Sullivan [1999] patented androgen analogues or a therapeutically effective analogue of TGF beta and measured increased tear levels of TGF beta. He noted that in Sjögren's syndrome there are lymphocytic infiltrations into the main and accessory lacrimal glands, an immune mediated extensive destruction of lacrimal acinar and ductal tissues that leads to KCS. He cited reports that cyclosporin (CsA) or glucocorticoids are ineffective in this condition and may worsen the disease (though recent articles support their effectiveness). He stated that androgens can help both meibomian and lacrimal gland dysfunction and reduced lymphocytic infiltration in Sjögren's syndrome based on tests on mice.
Bodor's patent [1999] lists the formulation of an eyedrop containing androstene derivatives with anti-inflammatory action.
Estrogens. Decreased estrogen levels can dry mucous membranes. Lubkin [2000] reported the effects of 0.1% and 0.25% of estradiol in oil vs the vehicle in a study with 44 post-menopausal dry-eyed subjects. She noted strong improvement of symptoms and signs from estradiol treatment. Figure 4 shows a dose-related improvement of tear break-up time (TBUT). Akramanian et al (1998) reported on a one-week course of treatment with estrogen ointment in 22 postmenopausal women. They reported a statistically significant increase in Schirmer and TBUT values, but no improvement in rose bengal scores. These reports were occasionally negated where no estrogen receptors had been identified in associated ocular tissue. The recent report by Esmaeli et al (2000) of estrogen receptors in meibomian tissue suggests dual causation of dry eye conditions associated with decreased post-menopausal estrogen levels.
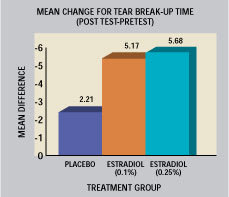
Figure 4. Effects of instilled estradiol in oil vs
the vehicle on TBUT in post-menopausal dry-eyed
subjects. Lubkin U.S. Patent [2000]
Foulks (1998) suggested that a combination of estrogens and androgens could well provide a salutory, additive effect in dry eye treatment.
Other Lacrimal Gland Components
Growth factors. Corneal homeostasis requires lacrimal gland derived growth factors; a stable preocular tear film requires a normal corneal epithelium. Epidermal growth factor (EGF) has been applied locally with success, especially post-keratoplasty, to enhance surface epithelial tissue.
Wilson et al (1999) postulated that a decrease in hepatic growth factor (HGF) would decrease tear film adherence. They reported that the concentration of EGF and HGF in normal tears increases in response to neural stimulation. Feedback from the surfaces modulates their concentration, and cholinergic stimulation increases secretion of EGF and lactoferrin. These authors suggested use of HGF in KCS. Formulation of a clinically useful preparation has the problems of source, purity and stability.
Lipocalin. Lemp (1999) and Mathers et al (1998) agree on the importance of lipocalin, or tear specific prealbumin. Present in high concentrations in normal lacrimal gland fluid, lipocalin has an affinity for lipids like Vitamin A and also may be vital in establishing a good interface between the lipid and aqueo-mucinous layer. Mathers also opined that lipocalin interacts in concert with tear mucin and proteins.
Natural Products
Autologous serum. Variable results in treating refractory KCS with autologous serum may be related to sample preparation and storage.
Tsubota et al (1999) diluted serum to 20 percent with saline in treating persistent epithelial defects associated with dry eyes. They assayed refrigerated and frozen material at one and three months for Vitamin A, EGF and TGF-beta and found them stable. Using response of persistent epithelial defects as end points, they reported approximately 44 percent healed by two weeks and 63 percent by one month.
Human serum albumin. Tsubota [2000] patented human serum albumin use for corneal and conjunctival epithelial problems including KCS. The concentration ranges from 1 mg/ml to 1,000 mg/ml. Reportedly this treatment also enhances mucin production.
Stem cell factor. Ogawa et al [1999] advocated topical application of a stem cell factor (HSF), administered locally to promote healing, listed primarily for corneal epithelial defects. Treatment consists of human stem cell factor, a product of recombinant prokaryocyte or eukaryocyte cells and a non-natural glycosylated polypeptide similar to HSF.
To receive references via fax, call (800) 239-4864 and request document #69. (Have a fax number ready.)
*Note: Dates of patents are in brackets [ ] and dates of scientific papers are in parentheses ( ).




