CONTACT LENS SOLUTIONS
Solution Leaps the Regulatory Hurdle
By
Sharon W. Lambert, BS, Karen Lindley, MBA, Mary Mowrey-McKee, PhD
March 2001
Let's see how a four-hour care product moves to a 10- minute regimen.
With the launch of CIBA Vision's Solocare as a 10-minute contact lens care product, some have wondered how a four-hour disinfecting regimen product is labeled as a 10-minute regimen with no chemical component changes. To answer this question, we must turn to the FDA 510(k) Guidance Document for Contact Lens Care Products (May 1, 1997) and ISO/DIS 14729 Contact Lens Care Products Microbiological Requirements and Test Methods for Products and Regimens for Hygienic Management of Contact Lenses (rev. April 1999). A lens care product requiring a regimen (a rub-and-rinse step) such as Solocare must successfully meet secondary Stand Alone Test criteria as well as Regimen Test criteria.
Environmental vs. Clinical Organism Derby
Both tests challenge a disinfecting product with a standardized inoculum. The species used as inoculum are representative of the range of pathogens a patient may encounter in everyday lens wear. Table 1 describes the organisms commonly used in Stand Alone testing.
The organisms listed in Table 1 were obtained from the ATCC repository in Maryland where their growth conditions have been strictly documented and monitored. Additional acceptable repository sources are supplied in both the ISO and FDA 510(k) guidelines. However, because the FDA guidelines list only one other source for each organism and the ISO guidelines refer to other sources being "equivalent to ATCC," only the ATCC strains are mentioned in this article. Once any organism is cultured in laboratory conditions (controlled environment lab-prepared media), the organism may undergo changes and therefore may exhibit a different pattern of resistance or susceptibility. However, the guidelines stipulate that test organisms can be no more than five passages (subsequent cultures) from the original ATCC repository stock. This reduces the variability in the results between laboratories and from one test date to another in a single laboratory.
The amount of inoculum used in both the Regimen and Stand Alone tests is not intended to be representative of the amount of microbial challenge that the patient would be exposed to. Rather, the amount of inoculum used is intended to be high enough to provide countable numbers from which an estimation of the solution kill rate can be determined. Additionally, the test inoculation levels, 100,000 to 1,000,000 colony forming units (cfu)/ milliliter, is higher than that thought to be commonly experienced by a contact lens patient. Tables 2-4 demonstrate other researchers' findings concerning actual recovery levels from lenses and the surrounding area.
ISO and FDA Standards
Both the Stand Alone Test and Regimen Test require three separate product lots that are challenged with separate inoculum preparations in order to provide a well-rounded assessment of product performance. An average of the three lots is used to determine whether or not a product has met test criteria. Additionally, each test requires that the medium used to neutralize the test product, and thus stop the product's biocidal activity, be tested for effectiveness. Each neutralization medium must prove its ability to neutralize the test product to such an extent that a low-level inoculum (10 100 cfu/mL) would be recoverable.

As stated earlier, a regimen-type disinfectant product must meet secondary Stand Alone criteria as well as Regimen Test criteria. In the Stand Alone Test, a known amount of solution is challenged with a known amount of challenge organism and measures the amount of organism that has been eliminated at pre-determined time intervals comparable with those during which the product might be used. To meet secondary Stand Alone criteria, the sum of the average log reductions for the three bacteria must be equal to or greater than 5.0 logs within the recommended soaking period, and a minimum average log reduction of 1.0 logs must be obtained for any single bacterium. Stasis (no kill required and no growth) must be observed for yeast and mold.

The Regimen Test challenges the entire lens care regimen for the test product with the same challenge organisms as those used in the Stand Alone Test. Tests conducted for submission to the FDA require the use of organic soil mixed with the organism suspension. The inoculum is carried through the regimen by being applied to a contact lens. In carrying out the Regimen Test procedure, the products are used in the manner and quantity recommended in product labeling and/or patient instructions. The lens and the entire volume of each lens case/well are cultured for viable microorganisms. In order to meet the Regimen Test criteria, the average regimen recovery count for all three lots tested must be 10 or less cfu per lens/storage solution combination for each lens type tested. Data from more than one lens type may not be combined to calculate the average.

Hitting the Mark
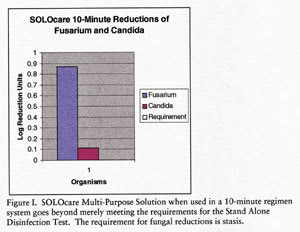
Solocare Multi-Purpose Solution exceeded the secondary Stand Alone Test and Regimen Test criteria with a 10-minute disinfection period. Figure 2 demonstrates that Solocare 10-Minute reduced all bacterial loads in the Stand Alone Test beyond the minimal 1.0 logs, as well as surpassing the 5.0 combined log reduction requirement (11.4 combined log reduction total). Solocare also demonstrated some fungal kill where stasis was the requirement (Figure 1). Having exceeded secondary stand alone test criteria, Solocare was evaluated in the Regimen Test using Group I (tefilcon) and Group IV (vifilcon A) lenses according to the Regimen Test. The test criteria were met with a regimen consisting of a 10-second rub and rinse per lens side, followed by a 10-minute soak in Solocare. As Figure 3 demonstrates, the Solocare regimen eliminated more than 199,990 organisms from each test lens. Test lenses were inoculated with a minimum of 200,000 organisms per lens and the average number of organisms recovered from the test group was < 10 per lens/solution combination.


The Stand Alone Test is designed to qualify individual solutions with clear antimicrobial activity as contact lens disinfection products. The Regimen Test is designed to qualify individual solutions as a part of a contact lens disinfecting regimen. Products meeting the regimen test criteria must also meet the minimum performance requirements of the Stand Alone Test. Ocular toxicology concerns, process convenience and product comfort on the eye have resulted in an evolution of products which maintain a low incidence of contact lens-associated ocular infection when used as instructed by the manufacturer.
Solocare is an excellent example of how an appropriate multi-purpose solution, when used with a good lens care regimen, can be very efficacious in eliminating harmful environmental threats to contact lens patients. Solocare, when tested with a 10-minute soaking period in the Stand Alone Test, more than fulfilled the secondary Stand Alone Test criteria. When the Solocare solution was tested as a part of a regimen system (with a manual rub-and-rinse step) in the Regimen Test, the regimen again more than fulfilled the Regimen Test criteria. Thus, the Solocare Multi-Purpose Solution lens care system using a 10-minute soak is an effective way to maintain ocular health for contact lens patients.
To receive references via fax, call (800) 239-4684 and request document #69. (Have a fax number ready.)
Mary
Mowrey-McKee, PHD, is the group leader of Toxicology and Microbiology at
CIBA Vision Corporation and is an expert on the ISO Working Group Developing
standards for Contact Lenses and Lens Care Products.
Sharon W. Lambert, BS, is a microbiologist in
the Lens Care Development Department at CIBA
Vision.
Karen Lindley, MBA, is Director of Lens Care Development at CIBA Vision.



