continuing education
Preventative Contact Lens Care: Part II
BY MARJORIE J. RAH, OD, PHD, AND RONALD K. WATANABE, OD
May 2001
This second installment of a three-part series explores contact lens fitting and prescribing, as well as replacement schedules and complications.
Part I of this three-part series on preventative contact lens care appeared in the February 2001 Contact Lens Spectrum and discussed the preliminary evaluation of prospective contact lens patients. Part II of this article continues from there. It describes the special emphasis placed on slit-lamp biomicroscopy of the eyelids, conjunctiva, cornea and precorneal fluid, which comprise the host environment for contact lenses. Initial prescribing considerations, including the very important lens replacement schedules, and fitting are discussed. The article then describes ocular tissue reactions and their prevention and remediation. Part III of this series (August 2001) is devoted to an overview of contact lens solutions from patient, in-office, FDA and Center for Disease Control (CDC) perspectives
Slit Lamp Biomicroscopy
A thorough biomicroscopic examination of the cornea is an essential part of the contact lens examination. Diffuse illumination at low magnification (almost 10x) can be used to scan the eyelids and conjunctiva to detect meibomian gland dysfunction, conjunctival injection, tarsal abnormalities, hordeolum, entropion, ectropion and blepharitis. A parallelepiped at higher magnification (15x to 30x) using direct and retroillumination techniques will reveal many of the common findings associated with contact lens wear, such as neovascularization, corneal fluorescein staining, subepithelial infiltrates and microcysts. Once these conditions are detected, it is imperative to assess their severity in order to initiate appropriate treatment. Several grading scales are available for documenting ocular findings. Tables 1 to 3 provide examples of grading scales for neovascularization, infiltrates and microcysts.
TABLE 1 |
||
Grading Scale for Corneal Neovascularization |
||
| GRADE | SEVERITY | DESCRIPTION |
| 0 | None | None |
| 1 | Trace | Congestion and dilation of the limbal vessels; single limbal vessel extension <1.5mm from prefitting position |
| 2 | Mild | Extension of limbal vessels <1.5 mm from prefitting position |
| 3 | Moderate | Extension of limbal vessels 1.5 to 2.5mm from prefitting position |
| 4 | Severe | Segmented or circumscribed extensions of limbal vessels more than 2.5 mm inside the limbus or extension to within 3.0 mm of pupil center |
|
TABLE 2 |
||
Grading Scale for Subepithelial Infiltrates |
||
| GRADE | SEVERITY | DESCRIPTION |
| 0 | None | None |
| 1 | Trace | Few faint infiltrates |
| 2 | Mild | Multiple or dense |
| 3 | Moderate | Marked infiltrates with overlying staining |
| 4 | Severe | Corneal Ulcer |
|
TABLE 3 |
||
Grading Scale for Epithelial Microcysts |
||
| GRADE | SEVERITY | DESCRIPTION |
| 0 | None | None |
| 1 | Trace | Less than 10 |
| 2 | Mild | 10 to 50 |
| 3 | Moderate | More than 50 |
| 4 | Severe | Numerous dense coalesced microcysts with significant staining |
Precorneal Fluid
The precorneal fluid smooths the anterior corneal surface, rinses away debris, provides gas exchange, maintains the pH of the tears, combats infection and wets the eye. When a contact lens is placed on the eye, the precorneal fluid maintains hydration and holds the contact lens in place. It is made up of three layers: the lipid layer, the aqueous layer and the mucoid layer. The meibomian glands and the glands of Zeiss and Moll produce the outermost lipid layer. The main lacrimal gland and the glands of Krause and Wolfring produce the middle aqueous layer. Finally, the glands of Manz, goblet cells and the Crypts of Henle produce the innermost mucin layer. Deficiencies in any layer can produce dry eye symptoms. Aging, blinking abnormalities and corneal epithelial abnormalities can also cause dry eye symptoms. These symptoms can be exacerbated by contact lens wear, which disrupts the normal structure and thickness of the tear layer. Patients with dry eye will present with symptoms such as burning, grittiness, itching or dryness. Ocular irritation may also result in complaints of excessive reflex lacrimation.
There are several diagnostic tests for dry eye. Evaluation of the tear prism, fluorescein tear break-up time, fluorescein staining of the cornea, rose bengal staining, lissamine green staining, Schirmer test and the phenol red thread test are a few of the most common. Table 4 provides a list of normal values for clinical dry eye tests. In addition, although not routinely performed in a clinic setting, lactoferrin analysis and tear osmolarity can be measured. While no one test is ideal for diagnosing dry eye, a positive test on one or more along with patient-reported symptoms increases confidence in the dry eye diagnosis.
|
TABLE 4 |
|
|
Normal Values for Dry Eye Diagnostic Tests |
|
| Diagnostic Test | Normal Value |
| Tear prism evaluation | Approximately 1mm |
| Fluorescein tear break-up time | Greater than 10 seconds |
| Schirmer Test | Greater than 5 mm/5 min |
| Phenol Red Thread Test | Greater than 10 mm/15 sec. |
|
TABLE 5 |
|
FDA Hydrogel Lens Categories |
Group 1: low water (<50%), nonionic |
Group 2: high water (>50%), nonionic |
Group 3: low water (<50%), ionic |
Group 4: high water (>50%), ionic |
Management of dry eye usually begins with artificial tears. Although many options are available, preservative-free drops are better for contact lens wearers because such drops reduce the possibility of preservative absorption into the contact lens and subsequent hypersensitivity reactions. If artificial tears are not enough to alleviate dry eye signs and symptoms, punctal occlusion can be performed. Dissolvable collagen plugs determine the efficacy of the treatment prior to more permanent silicone plug insertion. Treatment of underlying conditions such as blepharitis or mebomianitis is also important to alleviate dry eye symptoms.
Initial Fitting and Prescribing Considerations
Materials and Deposits. Soft contact lenses can be divided into four Food and Drug Administration (FDA) hydrogel lens material categories (Table 5). High water content lenses have a greater propensity for lens deposition. Similarly, ionic lenses are more prone to surface deposition than non-ionic lenses. These deposits place patients at a higher risk for complications, such as contact lens-induced papillary conjunctivitis (CLPC) and other possible complications.
Many soft contact lenses combine materials whose permeability (Dk) and thickness (L) produce transmissibility (Dk/L) that is sufficient for daily wear. This level of transmissibility maintains the availability of oxygen to and dissipation of carbon dioxide from the cornea at levels that do not usually create the clinical signs or symptoms of hypoxia or hypercapnia. This is less true for extended wear, in which there is usually deficient oxygen supply in relation to the demand, thus increasing the probability of ocular tissue complications.
While most disposable or planned replacement contact lenses do not change the hypoxia situation compared to conventional soft contact lenses, they do reduce many of the inflammatory reactions due to their frequent replacement and lessening of deposits. Similar materials are typically used for conventional, disposable and planned replacement contact lenses; however, the newer silicone hydrogel lenses provide a much higher level of oxygen permeability than any current hydrogel lenses. Silicone hydrogel lenses and rigid gas permeable lenses are better suited for extended wear because their high oxygen transmissibility reduces the hypoxic conditions associated with continuous wear.
|
|
|
|
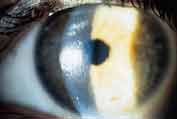
Figure 1. Coated lens worn by a patient with contact lens induced papillary conjunctivitis. |
Debris and elements of the tear film can deposit on the front or back surface of a contact lens. These deposits can be organic (proteins and lipids) or inorganic (calcium salts and iron) in nature (Figure 1). Mixed deposits such as mucoprotein-lipid complexes (jelly bumps) are also possible. Although not commonly thought of as lens deposits, microbial organisms can adhere to the contact lens surface as well. Patients wearing lenses with surface deposits may present with complaints of reduced vision, redness, poor comfort or tearing. Upon examination of the lenses, a film or discrete deposits will be observable along with possible discoloration.
Proper lens care and regular replacement schedules are essential in maintaining a clean contact lens surface. Poor compliance with either can lead to contact lens-related ocular complications. Unfortunately, patients often do not comply adequately, especially with conventional soft lenses.
Lens Replacement Schedules. Disposable and planned replacement soft lenses and multi-purpose solutions (MPS) have simplified lens care. Many contact lens-induced complications can be prevented simply through more frequent lens replacement and better compliance with simpler care systems. Replacement schedules with soft contact lenses can vary from daily to yearly. Assuming good patient compliance, daily to two-week replacement cycles are often the best preventative schedules to reduce ocular complications. This, in combination with properly prescribed and followed wearing schedules, patient education and ongoing care, is the best way to prevent adverse affects.
Fitting. Careful lens fitting and appropriate prescribing of soft lens material, design and replacement schedule are essential to produce and maintain good vision, comfort and ocular tissue integrity. For the best results, disposable and planned replacement lenses should fulfill the same criteria of position, movement and relationship between the back surface of the contact lens and the front surface of the eye as required for a good fit of any soft contact lens. However, many of the disposable lenses are thin low modulus lenses that will move less in primary gaze than many conventional lenses. The "push-up" test may be necessary to determine whether the lens is adhered to the cornea or will move freely.
When fitting extended wear lenses, you are more likely to see post-lens debris and lens adherence to the cornea in the morning. Instruct patients to use lubricants upon awakening and prior to lens removal. Lenses should begin to move freely within 30 minutes of waking, and debris should be able to be flushed from under the lens during waking hours.
Complications
|
|
|
|
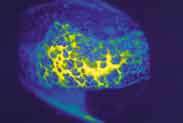
Figure 2. Contact lens-induced giant papillary conjunctivitis. |
|
Contact Lens-Induced Papillary Conjunctivitis. Giant papillary conjunctivitis (GPC), also known as contact lens-induced papillary conjunctivitis (CLPC) in the earlier stages, is a chronic inflammatory response of the palpebral conjunctiva. It is thought to be a result of Type I and Type IV hypersensitivity reactions to protein deposition on the contact lens surface. This reaction is most likely due to mechanical and immunologic irritation of the palpebral conjunctiva. It is typically a bilateral condition.
In early stages of the condition, patients will report symptoms of itching, foreign body sensation and mucus discharge. As the condition progresses, contact lens tolerance will decrease. Biomicroscopic changes in the superior palpebral conjunctiva begin with hyperemia. As the condition worsens, papillae form. If left untreated, the papillae can grow in size to greater than a millimeter in diameter. The presence of these "giant" papillae can cause the contact lens to position superiorly and/or move excessively (Figure 2).
Management of the condition begins with discontinuation of lens wear. Subjective symptoms will resolve before slit lamp findings diminish. CLPC is a chronic condition, and symptoms often return once lens wear is resumed if no changes in lens replacement schedules and/or care systems are made. More frequent replacement of contact lenses will lessen the amount of deposition on the contact lenses and will help to reduce recurrent episodes. Daily disposable or two-week disposable lenses are ideal in the management of contact lens-induced papillary conjunctivitis. Enzymatic cleaning is recommended with less frequent replacement of the lenses. In all cases, compliance with the care regimen is essential.
In moderate to severe cases of GPC, you may need to include therapeutic intervention in the form of anti-allergy and anti-inflammatory medications. Topical pharmaceuticals such as mast cell stabilizers, antihistamines, corticosteroids and non-steroidal anti-inflammatory (NSAIDs) drugs have all been shown to alleviate the signs and symptoms of the condition. Chronic use of corticosteroids should be limited only to the most severe cases of GPC as side effects such as increased intraocular pressure and cataract formation may result.
Patients who suffer from severe cases of GPC often are never able to comfortably return to contact lens wear. For this reason, prevention or early detection and treatment is critical. Perform lid eversion on all soft lens patients to detect early signs.
|
|
|
|
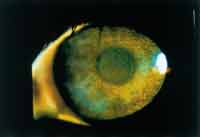
Figure 3. Superficial punctate keratitis caused by chemical toxicity. |
|
Superficial Punctate Keratitis. Superficial punctate keratitis is a common finding in both soft and rigid contact lens wear. It can be a result of mechanical irritation from scratched or torn lenses, chemical toxicitiy and hypersensitivity, dryness, contact lens overwear and/or buildup of deposits (Figure 3). In rigid lens wear, it may also result from inappropriate edge lift. Adjustments in the rigid lens design or prescribing disposable and planned replacement soft lenses can alleviate these problems and reduce the incidence of superficial punctate keratitis. More frequent replacement of contact lenses reduces the amount of buildup of deposits and reduces the absorption of chemicals from solutions. In addition, the multi-pack lenses make replacement of scratched or torn lenses more convenient for patients.
Contact Lens Overwear. Although contact lens manufacturers are making great strides in soft lens materials to increase Dk/L, contact lens overwear can still be problematic. Patients experiencing contact lens overwear will typically present with complaints of redness, poor comfort with contact lenses, photophobia and pain. Ocular signs can include conjunctival injection, limbal injection, edema, corneal distortion, spectacle blur and epithelial microcysts.
When hydrogel lenses are worn for long continuous periods, the oxygen demands of the cornea are not met. The cornea receives most of its oxygen from the atmosphere when the eye is open. Oxygen is necessary for aerobic glycolysis via the Kreb's Cycle. This process provides energy for the normal function of the cornea, tissue replication, growth and maintenance. When the cornea is deprived of oxygen by the contact lens for long periods of time, the cornea no longer produces enough energy to carry out its normal functions. Under such conditions, the cornea shifts to anaerobic metabolism, causing lactic acid to accumulate. This lowers the corneal pH, which can affect epithelial and endothelial integrity. The accumulation of lactic acid causes a change in the osmotic gradient and leads to edema. Clinically, edema with soft lenses will appear as posterior striae or folds and superficial punctate keratitis.
Epithelial compromise as a result of hypoxia often takes the form of microcysts. Microcysts appear as small whitish spots with bright direct illumination and show reversed illumination with indirect light. Although they are usually found in the midperipheral cornea, they may also appear centrally. Microcysts are comprised of apoptotic cells that migrate anteriorly through the epithelium and may stain when they reach the surface.
If the corneal endothelium is compromised, the result is corneal stromal swelling or edema. When the corneal swelling reaches approximately 6 percent, striae can appear. These will appear as thin white lines in the posterior stroma. In more severe instances (10 percent or more swelling), stromal folds may be noted.
Management of overwear involves decreasing wearing time and using a lens material with a higher Dk/L. If the patient suffers from acute onset with notable symptoms, temporarily discontinue the lenses until the eye is white and quiet. Switching patients from an extended wearing schedule to a daily wear schedule will reduce the hypoxic response.
Contact Lens-Induced Microbial Ulcerative Keratitis. The most sight threatening of all contact lens complications is microbial ulcerative keratitis, which fortunately has a low incidence. Although all contact lens wearers are at risk for developing infectious keratitis, some are more at risk than others. Extended wear has been implicated in a much higher incidence of infectious keratitis than daily wear. New materials such as silicone hydrogels are seeking to reduce this problem by increasing the Dk and Dk/L. Hypoxia due to low Dk/L and long wearing times also places a patient at higher risk. Poor compliance with care systems and replacement schedules can also place a patient at higher risk. Lens cases that go unattended and are left as breeding grounds for bacteria are often implicated in infectious keratitis. In addition to these risk factors, blepharitis and epithelial trauma are factors. What each of these risk factors has in common is the potential to compromise the corneal integrity and provide optimal conditions for microbial invasion. Although it can occur, microbial invasion of an intact cornea is rarely seen. A careful case history is necessary to elicit the presence of these risk factors and determine the causative agent as treatment depends on the type of microbial insult.
|
|
|
|
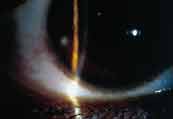
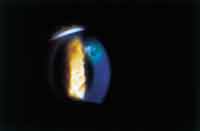
Figure 4. Peripheral ulcer secondary to hydrogel contact lens wear. |
Patients with microbial ulcerative keratitis present with symptoms of pain, photophobia and mucus discharge. Decreased vision may also be reported if the area of corneal insult involves the visual axis. Upon examination of the corneal surface, an infiltrate with an overlying epithelial defect that stains with fluorescein will be present (Figures 4 and 5). Corneal edema surrounding the lesion may also be noted. In moderate presentations, conjunctival edema and injection will also be evident. In the most severe cases, lid inflammation, anterior chamber reaction and hypopyon can also be seen.
Instruct patients to discontinue contact lens wear if they have not already done so. Although initial therapeutic intervention may be used immediately, take appropriate cultures to determine the microbe responsible for the infection, which may change the therapeutics used. Supplies for culturing can be obtained from a local laboratory or patients can be referred for this procedure.
|
|
|
|
Figure 5. Initial presentation of a Pseudomonas ulcer. |
Some of the most common sources of bacterial infection include: Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus pneumoniae, and Haemophilus influenzae. Extremely dangerous are those that can penetrate the intact cornea such as Neisseria gonorrhea, Corynebacterium diphtheriae, Haemophilus aegyptius and Listeria. It is also important to rule out fungal and acanthamoeba infections. Once you obtain the culture, begin treatment of the suspected source of infection. If you suspect a bacterial infection and small infiltrates are present, inititate a broad-spectrum topical antibiotic, such as a fluoroquinolone, every 15 to 30 minutes for the first six hours, followed by instillation every hour. The patient should return in 24 hours for a follow-up evaluation. For larger ulcers, fortified antibiotics are necessary. Once the results of the culture are obtained, adjust treatment accordingly. A topical cycloplegic can be used to increase patient comfort in moderate to severe cases. Use of topical steroids and eye patching are contraindicated.
The prognosis of microbial ulcerative keratitis depends on the size, location and severity of the infection as well as response to treatment. Corneal scarring will be present upon resolution. If the visual axis is involved, loss of best-corrected visual acuity will probably occur.
Changing from extended wear to daily wear can decrease the risk of microbial ulcerative keratitis. Decreasing the amount of time lenses are worn on a daily basis will improve corneal recovery. Compliance is an important issue to review with patients. Using the correct care system, adhering to prescribed wearing and lens replacement schedules, and proper care of lens cases also can decrease the potential for infection. Treatment of underlying conditions such as blepharitis will also decrease the risk of infection.
Contact Lens Acute Red Eye (CLARE). Contact Lens Acute Red Eye (CLARE) is an acute inflammatory response seen in soft contact lens patients, extended wear especially. The condition is typically unilateral. Patients frequently present with complaints of severe pain, photophobia and excessive tearing in one eye. Often, the patient will report being awakened in the early morning by the pain. A history of tight-fitting soft contact lenses is also commonly noted. Upon examination of the patient, subepithelial or anterior stromal infiltrates are present in the midperipheral cornea along with conjunctival injection. CLARE may be a hypoxic condition brought about by extended wear of tight-fitting hydrogel lenses. Other causes include Gram-negative bacterial contamination and bacterial toxins trapped between the lens and the cornea.
Management of the condition begins with discontinuation of contact lens wear. Palliative therapy is used to provide comfort to the patient. Although topical corticosteroids can be used to reduce the inflammation, they should not be used alone due to the risk of bacterial infection. Use of a broad-spectrum antibiotic such as topical fluoroquinolones or aminoglycosides during the first 24 to 48 hours is recommended. The inflammation will typically resolve after a period of several days to a few weeks. Lens wear should be resumed only after all signs of inflammation are gone. The patient should be refit into a looser-fitting lens and should be advised to wear the lenses on a daily wear schedule.
It is important to distinguish CLARE from infectious keratitis as they have similar clinical presentations. Both conditions can have an acute onset with conjunctival injection, excessive lacrimation and corneal infiltrates. However, no epithelial defects are noted in CLARE, while fluorescein staining over the infiltrative area will be present in infectious keratitis.
Prevention of recurrence can be achieved by recommending wearing lenses on a daily rather than extended wear basis. Reduce CLARE by frequently replacing contact lenses. Daily or bi-weekly lens replacement schedules reduce the amount of bacterial toxins on the lens as well as reduce the amount of lens deposits and absorption of preservatives from lens care systems. Avoid tight-fitting lenses. Perform follow-up visits toward the end of the day after the patient has worn the lenses to assure proper lens movement.
Summary
One common thread in the prevention of contact lens-induced ocular complications is prescribing daily and two-week replacement disposable soft contact lenses. When patients comply with prescribed and simplified care systems and wearing time, planned replacement schedules such as these provide good comfort and vision along with satisfactory corneal physiology, especially for daily wear.
Marjorie J. Rah, OD, PhD is an assistant professor and Ronald K. Watanabe, OD is an associate professor and Chief of Contact Lens Services at New England College of Optometry.
References are available upon request to the editors of Contact Lens Spectrum. To receive references via fax, call (800) 239-4684 and request document #71. (Have a fax number ready.)