MULTI-PURPOSE SOLUTIONS
Current Multi-purpose Solution Concepts
By Milton M. Hom, OD,
FAAO, and
Peter A. Simmons, PhD, FAAO
September 2001
The search for balance between efficacy and lack of ocular irritancy has driven the development of current MPS disinfectants.
A multi-purpose solution manufacturer faces many challenges. The chemicals that disinfect lenses in multi-purpose solutions must have sufficient anti-microbial activity, yet remain non-toxic to the eye. This balance between efficacy and lack of ocular irritancy has driven the development and selection of current MPS disinfectants.
The Disinfectant
Many solutions today have common components (Table 1). Current disinfectants rapidly bind to the microbe cell surface, causing a loss of membrane integrity, leakage of critical cytoplasmic components and cidal effect. PHMB (polyhexamethylene biguanide) is the most popular MPS disinfectant, and it is successfully used in MPS products around the world.
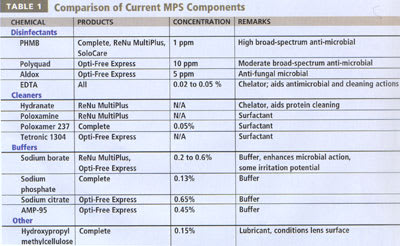
Allergan's Complete, Bausch & Lomb's ReNu MultiPlus and CIBA Vision's SoloCare all contain PHMB at one ppm. The biguanide group has a very strong cationic (+) charge that makes it highly active at very low levels. Other formulation ingredients (buffers, surfactants, salts, etc.) can affect its antimicrobial activity, which accounts for the differences in antimicrobial effectiveness between products. Overall, PHMB is an effective disinfectant that is well tolerated by most patients.
PolyQuad (Polyquaternium-1) is unique to Alcon products. It is a large cationic (+) polymer (larger than PHMB). The quaternary ammonium group has a lower cationic (+) charge than PHMB's biguanide group. Polyquaternium-1 is used at higher levels (10 ppm vs one ppm for PHMB).
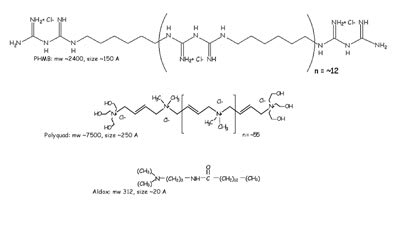
Opti-Free Express also contains Aldox at five ppm. Aldox, Alcon's trademark for myristamidopropyl dimethylamine, demonstrates anti-fungal activity. Aldox is a fatty acid modified with a cationic group. It is a much smaller molecule than PHMB and Polyquad (Figure 1).
|
|
|
|
Figure 1. Three current MPS disinfectant chemicals. PHMB and Polyquad are relatively large polymers, while Aldox is a small
molecule. |
|
No Rub and Stand Alone
There is no link between stand-alone testing and no-rub qualification, relative either to product performance or to FDA clearance. Many practitioners are confused in this regard, given that somewhat conflicting information is available to them. A recent article ("Contact Lens Solutions and Lens Care Update," June 2001) explains the differences between no rub and stand alone.
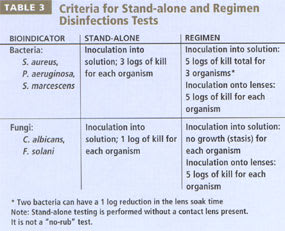
In the United States, there are two recognized ways to demonstrate that an MPS delivers the required disinfection performance. The first is regimen qualification, in which researchers artificially load lenses with micro-organisms, then perform each step of the specific regimen as specified in the product labeling. This testing must demonstrate that at the end of the disinfection process, fewer than 10 microbes of an initial inoculum of 100,000 to 1,000,000 remain on the lenses. The manufacturer specifies the regimen, which may or may not include a rub step.
The second method to demonstrate that an MPS meets disinfection requirements is stand-alone testing, developed to expedite industry testing of MPS products that have a "standard" regimen (rubbing and rinsing as well as a soak period). If the product passes the stand-alone test, regimen testing is not required, and the manufacturer may label the product as a multi-purpose disinfecting solution (MPDS) if desired.
|
|
|
|
Figure 2. Stand-alone antimicrobial testing. No contact lenses are used in this test. |
The FDA 510(k) document dated May 1, 1997, describes the current standard for stand-alone disinfection. Technically researchers soak three bacteria (S. aureus, P. aeruginosa, S. mar-cescens) and two fungi (C. albicans, F. solani) for the indicated time (typically four to six hours for an MPS) and then count the amount of surviving organisms. Passing stand alone testing does not demonstrate either cleaning or disinfection of lenses because no lenses are involved in the test (Figure 2). It requires only a specific reduction in microbial numbers, termed log reduction (see "What is 'log reduction'" below), within the specified soak time.
By contrast, regimen qualification is a more stringent demonstration of disinfection ability. It requires virtually complete disinfection of lenses in an actual simulation of patient use. Table 3 summarizes the differences between stand-alone and regimen testing.
Initially, Alcon's Opti-Free Express and Bausch and Lomb's ReNu MultiPlus both passed the stand-alone test. Allergan's Complete and CIBA Vision's SoloCare both passed the regimen (rub, rinse, soak and rinse) qualification.
|
|
What Is "log reduction?" |
|
"Log reduction" refers to the number of live organisms killed. For contact lenses, it signifies a disinfectant's effectiveness. Researchers typically use a large number of organisms for testing. This example shows 500,000 organisms. For stand alone, the product must kill 450,000 out of 500,000 organisms. For regimen qualification, the product must kill 499,995 out of 500,000 organisms to pass (See Table 2).
In the real world, 500,000 organisms do not reside on the lenses. Even in a heavily-contaminated lens case, the bacterial count is most likely 1,000 or fewer organisms. Researchers use a large inoculum in these tests to compare the relative performance of different products, and to provide an indication of performance under worst-case conditions. |
A no-rub regimen is not a "standard" regimen, so an MPS product must pass a modified regimen test to obtain FDA clearance for no-rub labeling. Currently, Alcon's Opti-Free Express has qualified as a no-rub MPS for all lens types, and Allergan's Complete has qualified as a no-rub MPS for frequent replacement lenses.
Is the stand-alone test "better" than the regimen qualification, either with or without a rub? The stand-alone test requires a three-log reduction of bacteria, while the regimen qualification (with or without a rub) requires a total of five logs of reduction. You could argue that the overall anti-microbial performance as demonstrated with a no-rub regimen is superior to that demonstrated in the soak-only stand alone test.
The Rinse Is the Key
With all of the talk surrounding no-rub and stand- alone, what has not been covered is the rinse step.
In the past, rubbing and rinsing (otherwise known as daily cleaning) were important to contact lens care. These steps remove grime from the lens as well as decrease the bacterial load, which allow a quicker and more effective disinfection cycle. The daily procedure to remove the bacteria adherent to the polymer surface included active rubbing for at least 20 seconds.
|
|
|
|
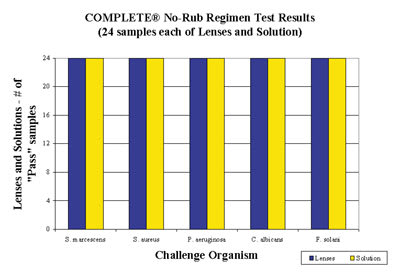
Figure 3. Results of no-rub regimen test for Complete MPS. "Pass" indicates >10 surviving
organisms. |
|
The rinse step is important for removing any residual cleaning agents. An inadequate rinse could cause foaming during enzymatic cleaning. For hydrogen peroxide systems, a poor rinse of surfactant cleaners could result in a bubbling action after neutralization. The solution could bubble out of the case and leave the patient with a dehydrated lens the next day.
The rinse step alone is highly effective in reducing the bacterial load. The cleaning or rubbing step removes one log unit of microorganisms. Adding a rinse removes two log units of contaminants.
Adequate rinsing alone typically removes at least 90 percent of the debris and contaminants from the lens. The disinfection action of the MPS then kills any remaining organisms and prevents new growth. After the rinse removes this 90 percent, the disinfectant has a much easier task of reducing the contaminant load. "No-rub" works because the lens has very few microbes, mostly from handling, on it when it goes into the case. A combination of rinsing and disinfection with fresh solution in a clean lens case should remove any remaining contamination.
When to Rub
Rubbing and rinsing should clean lenses better in terms of removing debris and deposits. However, it does add more microbes from the hands and increases the risk of tearing the lens. No rub has less potential to damage the lens and adds less contamination from handling. Although a heavy depositor may do better with a rub and rinse regimen, published clinical studies on no-rub regimens have demonstrated equivalent or better cleaning performance with no rub as compared with a standard rub regimen.
Rinsing is crucial for the success of a no-rub regimen. Without the rinse step, no rub is not as effective. A recent letter to the editor ("No-rub Convenience May Not Mean Compliance," July 2001) quoted industry data that indicates a significant number (33 percent) of patients do not rinse with standard rub regimens. Clearly, compliance with rinsing is key to success with a no-rub regimen. Before recommending a no-rub regimen, practitioners should carefully evaluate the patient's current level of compliance (inspecting the cleanliness of a lens case brought from home may provide an objective clue), and thoroughly explain the importance of adequate rinsing with the no-rub regimen.
Safe and Effective
All MPS solutions effectively disinfect lenses when used as directed. Figure 3 shows an example of disinfection performance in a no-rub regimen. MPS solutions have evolved dramatically in recent years, and we can expect further enhancements in the years to come. By simplifying lens care, no-rub enhances compliance and benefits most of our patients.

References are available upon request to the editors of Contact Lens Spectrum. To receive references via fax, call (800) 239-4684 and request document #74. (Have a fax number ready.)