CONTINUOUS WEAR CANDIDATES
Selecting the Best Candidates for Continuous Wear
FDA
approval studies and
a risk factor analysis help form a profile of
the best candidates for silicone hydrogel continuous wear.
By Robin L. Chalmers, OD, FAAO
It has been more than a year since the first U.S. commercial introductions of high Dk silicone hydrogel lenses for 30 nights of continuous wear. Practitioners in Europe, Australia and Latin America have been prescribing these lenses for continuous wear a few years longer. Reports from manufacturers estimate that over half a million patients are currently wearing silicone hydrogel lenses worldwide, with the majority on a 30-night continuous wear schedule. Another high Dk continuous wear lens has begun the clinical trial process. The 30-night continuous wear modality is no longer a futuristic concept. Patients interested in continuous wear are now seeking guidance, advice and fitting of these lenses. Information is available to help you select the best candidates for these new lenses to maximize their success.
Continuous Wear and the FDA
Each brand of lenses approved for 30-night continuous wear underwent careful scrutiny in large clinical trials to obtain FDA approval. You can easily access a wealth of information detailing results from these studies (www.fda.gov/cdrh). For CIBA Vision's study, 522 subjects (1,044 eyes) wore Focus Night & Day lenses in both eyes for one year, and in Bausch & Lomb's study, 610 subjects wore one PureVision lens in one eye and one control lens in the other for one year. A review of the clinical experience from these collective 1,654 eyes, as well as from the 385 combined subjects who discontinued these studies, provides a good base of knowledge for clinical decision making. Until practitioners gain their own individual clinical experience with these lenses, let's tap the collective wisdom as a starting point.
Many practitioners are unaware that the FDA also requires post-approval studies for all 30-night continuous wear lenses, both silicone hydrogel and high Dk gas permeable lenses. Specifically, the FDA requires each manufacturer that has thus far obtained approval to follow 5,000 wearers for one year to "assess the rate of microbial keratitis that occurred during the one-year follow-up." A post-approval study is by design more observational in nature; the patient isn't randomized to any treatment but is observed for adverse events while wearing the device in a setting influenced by the clinical marketplace. Results from post-approval studies on 15,000 wearers will help practitioners fine tune their clinical practice with these lenses in the future.
Research studies have consistently demonstrated that high Dk continuous wear lenses improve many ocular signs of hypoxia including overnight corneal swelling, flushing of the circumlimbal vessels, myopic creep, microcysts and neovascularization. Many patients experience relief from symptoms of dryness and discomfort with these lenses. The literature also cites inflammatory complications associated with high Dk silicone hydrogel lenses, ranging from inflammatory events such as corneal infiltrates to contact lens peripheral ulcers, at a rate from 1 to 5 percent annually. There has been one case series of microbial keratitis published.
In this article I will review the results of various FDA silicone hydrogel lens approval studies and a risk factor analysis of one of those studies to form a profile of the best candidate for continuous wear with high Dk silicone hydrogel lenses.
|
|
|
|
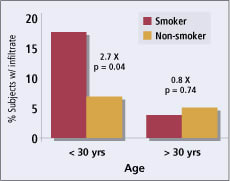
Figure 1. Effect of age and smoking on rate of inflammatory
events. |
|
Results of FDA Approval Trials
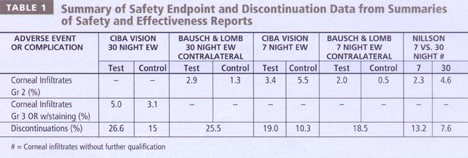
Each FDA Summary of Safety and Efficacy report revealed no significant difference in the rate of serious adverse events (defined slightly differently in each trial) when subjects wore high Dk silicone hydrogels for up to 30 nights compared to a control hydrogel lens for six nights (Table 1). The safety endpoints differed in that CIBA's endpoint included any infiltrate that had overlying fluorescein staining, while B&L's endpoint was limited to infiltrates of Grade 2 or higher. B&L also included a comparison of any Grade 2 or higher slit lamp findings. These studies had comparable results and show that a number of patients may experience some inflammatory episodes while wearing these lenses and that loss of vision related to these episodes is extremely rare.
The 30-night continuous wear studies had more discontinuations than the six-night trials. Approximately 25 percent of patients discontinued the 30-night continuous wear studies, many within the first month. Reasons included poor lens fit, discomfort, inability to tolerate extended wear and inflammatory event. Practitioners who can determine which patients are not suited for 30 nights of continuous wear will save chair time that is lost trying to steward a patient through an unsuccessful adaptation phase.
Significant Risk Factors
In analyzing the CIBA Vision FDA Registration Trial results, McNally et al explored a number of risk factors related to the safety endpoint of Grade 3 or greater corneal infiltrates OR any grade corneal infiltrate with overlying staining. We'll hereafter refer to these safety endpoint events as "corneal infiltrates." In previous studies, this endpoint had similar risk factors to those cited for microbial keratitis, so it was a good surrogate, or substitute, outcome that could be detected at a higher rate but that may indicate parallel risk for more serious inflammatory or infectious events.
Risk factors tested included smoking, ocular health history, previous lens type and wearing schedule, refractive error, gender, age, lens fit and occurrence of complications during the first month of wear. Some of these factors had been identified in earlier studies of complications from extended wear, while others were newly explored in this study. Because the study protocol focused on extended wear for up to 30 nights, the study could not evaluate increasing number of nights of wear as a risk factor.
This study of nearly 700 patients with lotrafilcon A lenses (Focus Night & Day) revealed no association between the corneal infiltrates and previous contact lens type (daily, disposable or GP), wearing schedule (extended wear or daily wear), refractive error, baseline neovascularization, gender or practitioner assessment of lens fit. Subjects with previous EW or DW experience showed no difference from new wearers. We found no increased risk in higher minus or plus lens powers compared to lower minus powers. There was no data on participation in water sports such as swimming.
The significant risk factors we identified were youthful subject age, smoking and ocular signs or history of inflammatory events.
Youth and Smoking Subjects aged 18 to 29 years were more than twice as likely to experience infiltrates, with 8.1 percent of subjects under 30 years of age experiencing events compared with 3.7 percent of those age 30 or older (odds ratio 2.2 times, p=0.018, Chi-square; n = 198 under 30 years) (Figure 1). Analysis of younger teenagers was not possible because the study population was limited to ages 18 or older.
The infiltrate event rate among all smokers in the trial (7.8 percent) was higher than for non-smokers (4.5 percent) but was not significantly different (p = 0.164, Chi-square; n = 103 smokers). However, when we analyzed the combined effects of smoking and age, the risk of events among smokers under age 30 was nearly triple the risk among non-smokers under the age of 30 (Odds ratio 2.7, p=0.034, Chi-square; n = 36 smokers under age 30 years) (Figure 1). Smoking did not impact the risk of events among subjects older than 30 years (p=0.74, Chi-square; n = 67 smokers age 30 and above) (Figure 1). If smoking alone caused some physiological impact on the smokers' immune system, the differential effect with age would not be so striking. Smoking in young people may indicate other risk-taking behaviors or a lack of respect for overall health that changes their susceptibility to inflammation with contact lenses.
|
|
|
|
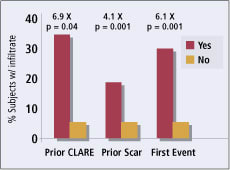
Figure 2. Effect of prior inflammatory events on rate of
inflammatory events. |
Prior Ocular Inflammation A history of prior inflammatory episodes at the baseline visit, such as a small corneal scar or a patient-reported history of acute red eye episodes, was also associated with a sharp increase in infiltrates in this trial. Some 18.5 percent of the trial subjects who entered the trial with corneal scars had infiltrates compared with 4.5 percent of the subjects without baseline scars (O.R. 4.1; p=0.001, Chi-square). History of previous red eye episodes translated into a nearly seven times higher risk of developing events during the study (p=0.0324, Fisher's Exact Test; n = 33 subjects with history of previous red eye) (Figure 2).
Of the 33 subjects who experienced an endpoint infiltrate, 10 (30.3 percent) had a second infiltrate endpoint before finishing the one-year study. Subjects who experienced a first event were nearly six times as likely to have a second event compared to the overall group of subjects (p=0.001, Chi-square). Over 40 percent of the infiltrate events occurred during the first month of wear.
How Do These Results Interact?
Patient selection in prospective clinical studies can unintentionally create subtle (or not so subtle) bias in results. Both of the two large FDA studies on silicone hydrogels had a mean subject age of approximately 34 years. In its 2001 Annual Report to Vision Care Professionals, Bausch & Lomb reported that the average age of lens wearers is substantially younger than this. Among patients requiring vision correction, 53 percent of those between the ages of 13 and 34 use contact lenses compared to only 32 percent of the 35 to 49 age group. For year-long prospective studies requiring eight or 10 visits, clinical investigators most likely choose more stable, reliable patients as potential subjects. The absence of many teens and college-aged people in the tested cohorts may have inadvertently failed to uncover a group of patients who, although interested in continuous wear, may be at higher risk for infectious complications or inflammatory events with the lenses.
A handful of cases of microbial keratitis associated with silicone hydrogel lens wear have been reported from around the world. Lim and Loughman reported one of the first case series that described four young men from Melbourne, Australia, all under 22 years of age. Three of the four young men had recently been swimming. Their smoking status was not reported.
The Best Candidates
I conclude from this research that the best candidates for 30-night continuous wear with silicone hydrogel lenses are non-swimmers over age 30 with no history of prior episodes of inflammation with lenses. Patients who now have neovascularization or have experienced myopic creep, contact lens-related dry eye or red eyes at the end of their wearing day will appreciate the relief from hypoxia these lenses offer. I would avoid 30-night continuous wear in young adults and teenagers who smoke and prescribe these lenses for shorter wearing periods for those patients. Patients who swim as a main form of exercise are advised to wear goggles in chlorinated swimming pools and to remove lenses when swimming in fresh water.
The occurrence of events early in the trials show that the first month of wear is an important follow-up period. If the patient cannot achieve comfortable, inflammation free wear in that month, recommend a shorter wearing schedule.
To receive references via fax, call (800) 239-4684 and request document #88. (Have a fax number ready.)
Dr. Chalmers is a clinical trial consultant with a particular interest in dry eye symptomatology and epidemiology issues with contact lenses.