Extreme Testing of Contact Lens Disinfecting Products
Multi-
purpose solutions face pathogens and environ-mental extremes to replicate
real world contact lens experience.
Ruth A. Rosenthal, MS, Cindy L.
Henry, BS, Sally L. Buck, BS,
William M. Bell, MS, Ralph P. Stone, PhD, and Barry A. Schlech, PhD
Patients are notoriously unreliable when it comes to properly caring for their contact lenses. Despite practitioner efforts to educate patients about proper lens care, non-compliance remains an unfortunate reality of clinical contact lens practice. The consequences of non-compliance are well known and include a host of contact lens complications, ranging from minor and transient to serious and sight threatening. In response, manufacturers have increasingly risen to the challenge of producing contact lens disinfecting products that are more effective and more convenient to use than predecessor products.
|
|
|
|
Figure 1. General antimicrobial activity of contact lens disinfecting products. |
|
Fear of infection and subsequent vision loss remains a primary concern with contact lens wear, perhaps out of proportion to the actual incidence of risk. Reports during the past decade associating microbial keratitis and overnight lens wear are prime examples. Micro-organisms from the environment, the normal flora of the eye and isolates from ocular infections may contaminate lenses and lens cases. Environmental sources of micro-organisms include water, air, soil, animals and plants. Lens cases are frequently cited in the literature as a source of contamination. Even handling of the lens may add potential contaminants. The degree of contamination may be related to compliance with patient instructions for use of the product. Nevertheless, the concern is real for both the patient and the contact lens community.
In the United States, the Food and Drug Administration regulates both contact lenses and lens disinfecting products. The antimicrobial activity of the product is assayed by a test (Stand Alone Test) that is designed to assess product performance using reproducible and quantifiable methods. The FDA permits contact lens products to be labeled as contact lens disinfecting solutions if they meet the primary criteria of the Stand Alone test for contact lens disinfectants against five representative challenge micro-organisms. This test uses no lenses, no soil and no rubbing or rinsing steps. Nevertheless, meeting the criteria of this test is proof of principle to the regulatory agencies that the product will perform as indicated during use. Based on historical data, it is assumed that if a product meets these criteria, then a disinfection system consisting of rubbing, rinsing and disinfecting with the product would also meet the criteria of the Regimen test, if tested. In contrast to the Stand Alone test, the FDA Regimen test is carried out with lenses in the presence of organic soil representing the components of the tear during consumer use. If the rubbing step is eliminated, then the product must be tested to determine effect of soil and tested by the FDA Regimen test to assure product efficacy during consumer use.
|
|
|
|
Figure
2. Effect of stored lenses on the antimicrobial activity of contact lens disinfecting
products. |
With the focus on meeting FDA guidelines and ISO standards, little attention has been paid to other performance criteria, especially those that challenge products with a variety of pathogens and environmental extremes that patients may encounter during their normal lens wear experience. Although logic suggests that disinfecting products be tested using organisms that have actually caused infection in lens wearers, such testing has generally not been performed.
In normal situations, contact lens-wearing patients may encounter countless varieties of micro-organisms, their contact lens cases may become contaminated, and their disinfecting products may not always be stored at temperatures called for by product labeling. Contact lens contamination can be significantly reduced when lens cleaning, rinsing, disinfection and the storage instructions are carefully followed. However, patients frequently skip the recommended rubbing and rinsing steps of their disinfection regimen. This is why it is critically important for products to retain the capacity to perform under extreme conditions and non-compliant patient behavior.
Comparative information regarding product disinfecting capacity and efficacy, especially in adverse real world situations, is limited. To address the need of both practitioners and patients for comparative data exploring real world performance of currently available contact lens disinfecting products, we designed a series of extreme challenges.
Extreme Testing
We tested four contact lens disinfecting products that are currently available in the United States. They were:
- Opti-Free Express Multi-Purpose Disinfecting Solution (Alcon), containing 0.001 percent Polyquad (polyquaternium-1) and 0.0005 percent Aldox (myristamidopropyl dimethylamine)
- ReNu MultiPlus Multi-Purpose Solution (Bausch & Lomb), containing 0.0001 percent polyhexamethylene biguanide (PHMB)
- SoloCare Multi-Purpose Solution (CIBA Vision), containing 0.0001 percent PHMB
- Complete Multi-Purpose Solution (Allergan), containing 0.0001 percent PHMB
A disinfection time of either four or six hours was used, depending upon the product's label.
|
|
|
|
Figure 3. Effect of high levels of Pseudomonas aeruginosa on the antimicrobial activity of contact lens disinfecting products. |
|
The media and reagents used for this study are described in the ISO 14729 standards for testing of contact lens disinfectants and were obtained from commercial sources. The challenge organisms used were Staphylococcus aureus ATCC 6538, Pseudomonas aeruginosa ATCC 9027, Pseudomonas aeruginosa, clinical isolate 6206, Pseudomonas aeruginosa, clinical isolate 6294, Serratia marcescens ATCC 13880, Candida albicans ATCC 10231 and Fusarium solani ATCC 36031. The specific organisms utilized for each test are described in the methodology. All Pseudomonas aeruginosa clinical isolates were obtained from Suzanne Fleiszig, OD, and Carol Lakkis, OD, of the University of California at Berkeley.
General Antimicrobial Activity
To evaluate standard antimicrobial activity and to establish an antimicrobial activity baseline, 10 ml of each product was challenged with 0.1 ml of approximately 1X108 CFU/ml of P. aeruginosa ATCC 9027, S. marcescens, S. aureus, C. albicans, and F. solani using a procedure based upon the FDA and ISO 14729 Stand Alone test (no rubbing, no rinsing and no lenses). The final concentration of the microorganisms in the product was approximately 1X106 CFU/ml. No organic soil was used in this study. The log reduction was calculated as [ Log10 (I) Log10 (F) ]; where I = initial concentration of organisms (CFU/ml) and F = final concentration of organisms (CFU/ml) after exposure to the product.
The primary criteria of the test requires greater than or equal to an average of a 3.0 log reduction of the three bacteria and greater than or equal to an average of a 1.0 log reduction of the two fungi at the label disinfection time. Results are shown in Figure 1. SoloCare and Complete multi-purpose solutions showed adequate bactericidal activity; however, both failed the primary criteria of the Stand Alone test against fungi because of insufficient kill. Both Opti-Free Express and ReNu MultiPlus met the primary criteria of the Stand Alone test. This means that these two products can be labeled as a contact lens disinfecting solution under FDA guidelines.
Contact Lenses Affect Antimicrobial Activity
Although not part of the disinfection equation in the FDA Stand Alone testing protocol, contact lenses can themselves interfere with antimicrobial activity of the products. To test this possibility, new Group IV soft, hydrophilic contact lenses (Vistakon Surevue) were soaked in all test products (ratio 2 lenses per 10 ml) for seven days. No organic soil was used in this study. The lenses were removed, and the "used" solution was tested for antimicrobial activity against P. aeruginosa ATCC 9027 and S. aureus. The percent log reduction was calculated as [ a / b * 100 ]; where a = log reduction of product with stored lenses and b = log reduction of product without lenses.
The test was created as an indirect method of determining how extensively the antimicrobial agent is taken up into the lens during storage and consequently, not available for disinfection. ReNu MultiPlus, SoloCare and Complete all showed a loss of antimicrobial activity after storage of lenses in the product (Figure 2). The bactericidal activity of Opti-Free Express was similar before and after exposure to lenses, suggesting that the antimicrobial components were not taken up into the lens, thereby allowing Opti-Free Express to maintain disinfectant activity in the presence of lenses.
|
|
| Figure 4. Effect of soil on contact lens disinfecting products. |
High Levels of Bacterial Contamination

Presence of Organic Soil
Soil can easily become a contaminant affecting contact lens care systems. To test any deleterious effects of organic soil, all products (10 ml) were inoculated with 0.1 ml of approximately 1x108 CFU/ml of the three Pseudomonas aeruginosa strains (ISO required strain [ATCC 9027] and two clinical isolates [6206 and 6294]) in the presence of laboratory prepared organic soil (killed yeast cells and inactivated fetal bovine serum). The final concentration of the micro-organisms in the product was approximately 1x106 CFU/ ml. The percent log reduction was calculated as [a/b * 100]; where a=log reduction of product with organic soil present and b=log reduction with no soil present. Opti-Free Express retained antimicrobial activity levels closer to baseline than any of the other products against all three P. aeruginosa strains (Figure 4). ReNu MultiPlus, SoloCare and Complete lost considerable antimicrobial activity against one or more of the strains.
Spectrum of Activity Against Clinical and Environmental Isolates
In the real world, contact lens disinfecting products may be subjected to a variety of potential pathogens. Beyond the representative FDA and ISO test organisms required for clearance to market, effectiveness against a broad range of microbes helps to insure patient safety. To explore its spectrum of activity, Opti-Free Express (10 ml) was challenged with 0.1 ml of approximately 1x108 CFU/ml using 60 different isolates. The final concentration of the micro-organisms in the product was approximately 1x106 CFU/ml. The isolates included 60 different species of Gram-positive and Gram-negative bacteria, yeast, mold, Acanthamoeba spp. and virus. Given the significance and relation to contact lens-related infection, 25 different strains of Pseudomonas aeruginosa, including both cytotoxic and invasive strains, were tested. Other products were not tested because of the magnitude of the study. Results are shown as log reduction at the label disinfection time (Table 1). The results confirm that Opti-Free Express has a high level of antimicrobial activity against numerous clinical and environmental isolates.
|
|
|
|
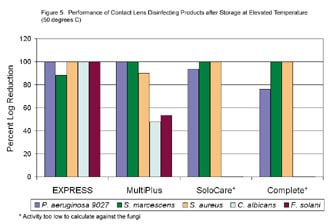
Figure 5. Performance of contact lens disinfecting products after storage at elevated temperature (50 degrees C). |
|
Storage at Elevated Temperature
Contact lens disinfecting products are not always stored under ideal conditions. To simulate extremes that may be encountered, all products were stored at elevated temperature of 50 degrees C (approximately 121 degrees F) for one week. Products were also stored at room temperature (approximately 25 degrees C) for one week as a control. The products (10 ml) were challenged with 0.1 ml of approximately 1x108 CFU/ml of P. aeruginosa ATCC 9027, S. aureus, S. marcescens, C. albicans, and F. solani. The final concentration of the micro-organisms in the final product was approximately 1x106 CFU/ml. No organic soil was used in this study. The percent log reduction was calculated as [ a / b * 100 ]; where a = log reduction of the product at elevated temperature and b = log reduction when stored at room temperature. The results show that the antimicrobial activity of Opti-Free Express was retained at elevated temperature (Figure 5). ReNu MultiPlus, SoloCare and Complete multi-purpose solutions maintained bactericidal activity at elevated temperature. However, ReNu MultiPlus showed less antifungal activity after storage at elevated temperature. The antifungal activity of SoloCare and Complete multi-purpose solutions was too low to evaluate. Opti-Free Express maintained similar antimicrobial activity compared to baseline against all five organisms when stressed by elevated temperature.
Conclusion
Patients and practitioners are faced with a variety of choices when selecting contact lens disinfecting products. Because each patient's wearing experience is unique, and exposure to a variety of pathogens and environmental challenges may occur, care systems should be assessed for their robustness and reserved capacity when subjected to extreme conditions.
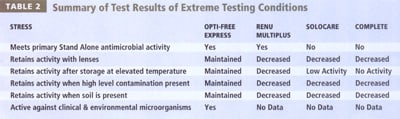
Of the products tested, Opti-Free Express demonstrated a higher capacity to kill micro-organisms under environmental stress or misuse conditions than did competitor products ReNu MultiPlus, SoloCare and Complete (Table 2). Moreover, SoloCare and Complete both fail Stand Alone criteria because of inactivity against fungi (yeast and mold).
Opti-Free Express performed beyond current standards for disinfection of contact lenses. In addition, it has the capacity to overcome a number of noncompliance issues that may occur in contact lens wearers. The extra capacity of the solution adds more assurance of effective product performance not only for non-compliant patients but also for compliant patients who face unexpected but nonetheless real challenges in their lens wear experience.
Ms. Rosenthal is the Director of R&D Consumer Products Microbiology at Alcon Research, Ltd.
Ms. Henry is a Scientist in R&D Consumer Products Microbiology at Alcon Research, Ltd.
Ms. Buck is a Senior Scientist at Alcon Labs in R&D Consumer Products Microbiology.
Mr. Bell is a Senior Scientist in R&D Consumer Products Microbiology at Alcon Research, Ltd.
Dr. Stone is the Vice President of Research and Development for Consumer Products at Alcon Research, Ltd.
Dr. Schlech received his microbiology degree from the University of Texas and is Alcon's Vice President of R&D Pharmaceutical Technology.