Medications and
Contact Lens Wear
The complex association between contact lenses and the effects of medications can influence
successful contact lens wear.
BY JOEL A. SILBERT, OD, FAAO
The relationship between contact lens wear and the effects of medications, either topical or systemic, is complex. There are considerable differences in how hydrogel and rigid contact lenses interact differently with topical pharmaceutical agents, based on fluid absorption and adsorption characteristics, the water content and thickness of hydrogel lenses, drug volume, the speed of drug concentration and release, and the retention time of the drug when in contact with the ocular surface. In addition, therapeutic agents, whether topically or systemically applied, can have adverse effects on the cosmetic appearance as well as the performance of a contact lens, or may produce greater adverse effects to the eye when administered in the presence of contact lenses.
|
|
|
|
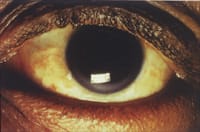
Figure 1. Very dry ocular
surface. |
|
Systemically-prescribed drugs may depress the central nervous system, and in turn may reduce blinking and subsequently affect tear pumping under rigid contact lenses, or may significantly decrease tear volume, inducing dry eye symptoms (Figure 1). For example, the use of alcohol, tranquilizers, barbiturates, some NSAID analgesics and muscle relaxants may lead to reductions in blink rate and blink amplitude. Many commonly-prescribed systemic medications can lead to ocular irritation, increased dryness, visual blurring and contact lens intolerance. Without a good understanding of these aspects of pharmacology, one is likely to quickly place blame on the contact lens material, lens fit or lens care products for the newly-encountered symptoms, which may not be actual cause of the problem.
Systemic Medications and Contact Lens Wear
The contact lens patient's medical history needs to be constantly reviewed and updated, as taking systemic medications may adversely affect his contact lens wearing time and tolerance. This is particularly true for the patient who has a long history of doing well with contact lenses but who now demonstrates symptoms of ocular irritation, increased dryness and overall lens intolerance.
Let's look at some examples of how systemic medications might influence contact lens wear.
Estrogens Women prescribed conjugated estrogens such as Premarin for vasomotor symptoms in menopause, hypoestrogenism, osteoporosis or atrophic vaginitis may report increased symptoms of dry eyes, increased corneal sensitivity, water retention including the possibility of corneal edema and contact lens intolerance. Switching to higher Dk lens materials is often helpful, as well as recommending increased use of preservative-free lubrication. Also consider adjunct therapy of punctal plugs.
Clinicians have long felt that oral contraceptives have been clinically implicated with contact lens wearing problems. It has been surmised that these agents may affect tear production, corneal curvature or corneal thickness. Unfortunately, despite these widespread anecdotal reports, there have not been well-documented studies in the medical literature to support a relationship between oral contraceptive use and complications with contact lens wear or contact lens intolerance.
Antihistamines Some patients may take antihistamines for ocular allergies year-round and others just during allergy season. For example, Claritin (loratadine), a long-acting tricyclic antihistamine with selective peripheral histamine H1-receptor antagonist activity is commonly prescribed for seasonal allergic rhinitis and idiopathic uticaria. Reductions in lacrimal secretion are common, resulting in a decreased aqueous volume in the precorneal tear film. In addition, there may also be reduced basal secretion in the accessory lacrimal glands. Antihistamines taken for seasonal allergies are typically H1-receptor antagonists. They not only are common causes of increased symptoms of dry eye but also may cause headache, drowsiness, dry mouth, conjunctivitis and ocular pain.
In addition, antihistamines are also commonly prescribed for the relaxation of stomach muscles. Drugs such as Zantac (ranitidine) are H2-receptor antagonists and may also produce similar symptoms in both contact lens wearers and non-lens wearers alike.
|
|
|
|
Figure 2. Disseminated corneal punctate
staining |
Beta-blockers This class of systemic drugs is widely used in patients suffering from supraventricular arrhythmias, angina and migraine headaches and is known to cause dry eyes. You are most likely to encounter these drugs when prescribed for control of migraine symptoms. Start the patient on ocular lubricants as soon as you know the patient is taking this category of medication. Additionally, beta-blockers might also lead to increased corneal punctate staining, so be sure to check lens wearers with sodium fluorescein after lenses have been removed, with a careful biomicroscopic examination (Figure 2).
Psychotropic medications More and more patients today are being prescribed medications to treat anxiety, depression and more severe psychiatric conditions. The benzodiapines, such as Valium, as well as the phenothiazines commonly cause increased dry eye symptoms. The serotonin reuptake inhibitors used to treat depression (Prozac or fluoxetine) have been used in patients of all ages, and may cause both dryness symptoms and well as reduced contact lens tolerance. Zoloft (sertraline), another serotonin reuptake inhibitor, is used to treat depression as well as obsessive-compulsive disorder (OCD) and panic disorders. In addition to a wide array of potential systemic side effects, Zoloft may cause dry mouth, eye pain, conjunctivitis and disturbed accommodation.
Anticholesterol medications With so many patients today suffering from obesity and elevated cholesterol levels, the contact lens practitioner is likely to encounter these drugs, particularly in the presbyopic population wearing or intending to wear contact lenses. Lipitor (atorvastin calcium) is an example of just such a medication used in the treatment of primary hypercholesterolemia. In addition, it is used to manage dyslipidemia and elevated serum triglyceride levels. Most side effects are minimal, and this drug is generally well tolerated. Patients may report increased headaches or muscle pain, and ocular symptoms include dry eyes and blurred vision, most of which occurs soon after beginning the medication. Manage dry eye symptoms with ocular lubricants and occasionally punctal occlusion.
Contact Lens Discoloration
Because of their ability to absorb water-soluble compounds, hydrogel lenses are subject to discoloration from a number of topical and systemic therapeutic medications. Topical drugs are absorbed directly into the soft lens. Systemic drugs discolor lenses by excreting into the tear film.
For example, although infrequently used today, epinephrine's oxidation products can cause hydrogel lenses to turn grayish-brown or black within two to six weeks after initiation of topical therapy. Phenylephrine and the systemic drug dopamine can also produce a similar discoloration from oxidation by-products (Figure 3). In addition to these "adreno-chrome staining" effects of lenses, darkly pigmented adrenochrome deposits can form in the palpebral conjunctiva, on the lid margin and rarely in the cornea from prolonged use of epinephrine.
Patients treated for urinary tract infections may develop orange-colored soft lenses as a result of excretion of phenazopyridine or nitrofurantoin in the tears. Laxatives containing phenolphthalein may stain hydrogel lenses pink or yellow. Tetracycline, a widely prescribed antibiotic, may cause some grayish-brown lens discoloration after prolonged use. This may coincide with the formation of dark brown to black granules in the palpebral conjunctiva, which are believed to be chelation complexes.
Rifampin, a systemic drug used in the treatment of tuberculosis as well as in meningococcal disease, can cause the excretion of orange or pink-colored tears, with resulting orange-colored soft lenses.
|
|
|
|
Figure 3. Grayish-brown soft lens discoloration. |
|
Topical Ocular Decongestants and Antihistamines
The societal as well as cultural emphasis on cosmetic appearance and the common misconception that a few blood vessels visible in the bulbar conjunctiva are signs of eye problems has often led patients to utilize vasoconstrictors instead of the safer category of ocular lubricants. This is especially notable in the contact lens-wearing population, where some increased hyperemia is typical and considered clinically normal (Figure 4). The occasional use of a topical ocular decongestant after lenses are removed is appropriate if the patient experiences mild itching or late-day redness. Topical drugs can persist within soft lenses, releasing the active ingredient over hours of lens wear. Thus, the use of ocular decongestants while wearing soft lenses should be avoided. The vasoconstrictive effects caused by these agents can mask hyperemia, ocular irritation and signs of a poorly designed lens such that the underlying cause of the problem goes undetected and untreated.
|
|
|
|
Figure 4. Significant superior limbal
hyperemia. |
The use of phenylephrine in low concentrations is widespread in many OTC ocular decongestants. Chronic use not only hides the cause of an underlying problem leading to hyperemia but also may create "rebound" hyperemia, analogous to the overuse of nasal sprays that can lead to even greater nasal congestion. Of even greater concern is the potential for phenylephrine-containing decongestants to induce pupillary dilation, which may be problematic for eyes with narrow angles. As corneal staining caused by ill-fitting lenses can lead to increased corneal drug penetration, the risk of angle closure glaucoma in predisposed patients is heightened with phenylephrine use.
In contrast to phenylephrine, the imidazole decongestants are safer to use with concurrent contact lens wear, without the rebound congestion effects. These drugs include naphazoline, tetrahydrozoline and oxymetazoline, with naphazoline being most widely used in ophthalmic topical decongestants. Occasional use (once or twice a day) of these agents while wearing soft contact lenses should present no problem for corneal integrity or for the lenses themselves. It is preferable for these agents to be used prior to lens placement and after lens removal. The patient should be advised to use them only when necessary, and never on a routine basis.
H1 histamine antagonists
Widely used in the treatment of ocular allergy of short duration, these drugs are typically found in combination with topical ocular decongestants as OTC agents. Examples include antazoline phosphate 0.5% (combined with naphazoline 0.05% in Vasocon-A, Novartis), and pheniramine maleate 0.3% (also with naphazoline 0.025% in Visine-A, Pfizer).
The first powerful topical histamine antagonist, Livostin (levocabastine HCl 0.05% ophthalmic suspension, Novartis) as well as Emadine (Emedastine difumarate 0.05% ophthalmic solution, Alcon) are indicated for the temporary relief of acute seasonal allergic conjunctivitis. They are not approved for use with soft lenses in place, but can be used prior to and after contact lens use.
|
|
|
|
Figure 5. Giant papillary
conjunctivitis. |
|
Topical Mast Cell Stabilizers and "Soft Steroids"
Clinicians have come to depend on mast cell stabilizers in the management of chronic mast cell-mediated ocular allergy, including vernal conjunctivitis and giant papillary conjunctivitis (Figure 5). These therapeutic agents prevent the release of histamine and other vasoactive mast cell constituents and also may protect the tear film from abnormal breakup. Cromolyn sodium 4% was the first such topical agent to be employed for this purpose and is still available as Crolom (Bausch & Lomb) and generically (Akorn). Currently, the indications for cromolyn sodium as well as for the mast cell stabilizers lodoxamide tromethamine 0.1% (Alomide, Alcon), and pemirolast potassium 0.1% (Alamast, Santen) are vernal conjunctivitis, vernal keratitis and vernal keratoconjunctivitis. Nedocromil sodium 0.2% (Alocril, Allergan) is approved for allergic conjunctivitis.
Despite these limited FDA indications, however, mast cell stabilizers are widely used clinically for GPC and chronic allergic conjunctivitis. Studies have shown that topical ocular application of cromolyn sodium in patients wearing hydrogel lenses does not result in significant drug accumulation in either daily wear or extended wear lenses. As many soft lens wearers are reluctant to discontinue contact lens wear in the presence of GPC, the use of disposable daily wear soft lenses alone or in combination with a mast cell stabilizer allows for both resolution of symptoms and gradual improvement in the signs of the disease without lens discontinuation. Perhaps the best choice for such patients is the use of a single use, one-day disposable hydrogel lens.
Today we have the added benefit of newer "multi-action" pharmaceuticals such as Patanol (olopatadine HCl 0.1%, Alcon Laboratories), Zaditor (ketotifen fumarate 0.025%, Novartis), and Optivar (azelastine HCl 0.05%, Bausch & Lomb). These drugs provide antihistaminic activity for control of the more acute, short-term allergic symptomatology as well as the long-term benefits of mast-cell stabilizer activity to control the underlying disease process, as they may be taken over several weeks to months. As patients may likely be wearing their contact lenses during this time period, these drugs can be easily prescribed bid for contact lens wearers. For more severe cases of acute papillary conjunctivitis that are not responsive to other therapies, the contact lens practitioner can also consider the benefits of the new "soft steroids."
In addition to the above medications used for control of allergic symptoms, there are times when the practitioner would like to use a topical steroid, but hesitates due to the multiplicity of potential side effects of these agents. For example, in cases of severely symptomatic GPC, when mast-cell inhibitors or multi-action agents may not bring rapid enough relief of symptoms, the use of a topical steroid is tempting, but it is generally inadvisable in the case of a soft contact lens wearer. With the advent of topical "soft steroids" such as Alrex (loteprednol etabonate 0.2%, Bausch & Lomb) and Lotemax (loteprednol etabonate 0.5%, Bausch & Lomb), the practitioner may choose potent agents that can effectively suppress allergic and inflammatory signs and symptoms, with many fewer side effects and risks of complications than with conventional topical steroids.
It has been shown definitively that loteprednol has a significant effect in reducing papillae in GPC patients who discontinued the use of their lenses during treatment. Other studies have shown that loteprednol rapidly and effectively reduces signs and symptoms of GPC, even when patients continued to wear their offending lenses. These agents are recommended for up to four times daily, instilling them prior to contact lens insertion and after lens removal. Although there is little chance of intraocular pressure elevation with the use of topical soft steroid usage, one should monitor IOP nevertheless. Under no circumstances should topical steroids of any kind be used concurrently with extended wear of contact lenses.
Medications Used With Bandage Contact Lenses
Managing patients with corneal abrasions traditionally has employed antibiosis, cycloplegia and pressure patching (Figure 6). Unfortunately, soft contact lens wearers who present with evidence of corneal abrasion have a greater likelihood of bacterial colonization on their lenses, which increases concern of the risks from pressure patching. This is more concerning when the history suggests that the patient has been wearing hydrogel lenses on an extended wear basis. A major drawback of patching is creating a warm environment for the incubation of pathogens, especially when frequent medication is needed. Consequently, for contact lens patients with corneal abrasions, the use of a soft bandage lens is often the preferred modality. An additional advantage of bandage lens therapy over patching is the visual benefit to the patient, who can see and function more comfortably. The lens eliminates the mechanical action of the eyelids over the newly forming and sliding epithelial cells.
The lens helps to break the pain cycle and allows for more rapid healing, as it eliminates the mechanical action of the lids over the newly forming and sliding epithelial cells. The bandage lens can be a low to medium water content hydrogel disposable that moves freely (but not excessively) during the blink or a silicone hydrogel lens.
|
|
|
|
Figure 6. Corneal
abrasion. |
Prior to placing the lens on the eye, instill topical antibiotic (fluoroquinolone or aminoglycoside), cycloplegic agent, as well as the NSAID ketorolac tromethamine (Voltaren, Novartis) for relief of pain and for its anti-inflammatory properties (as steroid use is generally contraindicated at this time). After the bandage lens is placed and checked on the eye, apply additional antibiotic and Voltaren drops, with periodic instillation prescribed throughout the day or into the next day, when the patient will be re-examined. As soon as the abrasion has been re-epithelialized, the bandage lens may be removed, with continuation or modification of therapy as indicated (a topical steroid-antibiotic drop might be substituted at this time to rapidly settle down persistent inflammation.) The use of hyperosmotic agents (sodium chloride 5% drops) are usually best started after initial re-epithelialization, to help in producing better tight-junctions between cells and with the hemidesmosomes.
The above scenario recommended for contact lens patients has also become popular in the general management of corneal abrasions in the general non-contact lens wearing population, with pressure patching becoming a less frequently-used treatment option.
Drug Delivery with Contact Lenses
Rigid lenses The use of rigid gas permeable lenses or PMMA lenses with topical ocular pharmaceuticals does not present any particularly problematic clinical issues, as these lenses do not absorb medications or preservatives. However, the presence of a rigid contact lens may impede adequate contact or spread of the drug over the corneal surface. Conversely, once under a rigid contact lens, there may be excessive contact time of the drug against the cornea, leading to greater likelihood of toxicity to corneal epithelial tissue.
The size as well as lens design characteristics may be responsible for these effects. An example of this effect might be the "reservoir effect" created with large diameter reverse geometry rigid lenses used in fitting patients after penetrating keratoplasty or after refractive sur-gery. This might also be true for some semi-scleral rigid lenses used in fitting advanced keratoconus. As some rigid lenses have the potential for inducing corneal edema, epithelial compromise and even reduced blinking, topical drug penetration may in fact be increased. For ordinary rigid gas permeable lenses, these effects are unlikely to occur, due to the smaller size of the lens and the excellent tear flushing which occurs.
While patients wearing rigid contact lenses can successfully use them while being treated with topical medications, it is generally best to instill medications without these lenses in place. Ointments are never appropriate in the presence of concurrent lens wear due to the likelihood of smearing the lens and disturbing vision. They are best applied before bedtime to the external lid structures or applied moderately to the cul de sac, as excessive ointment application can lead to residual blurring of vision the following morning. For patients with dry eyes who must wear contact lenses, the use of some of the newer gels rather than ointments is much preferred. The new gel products maintain good contact with the ocular surface during sleep but do not leave a residue when the patient awakens. Gels can also be used during the day with only brief periods of visual blurring.
Hydrogel lenses Unlike rigid lenses, hydrogel lenses do absorb fluids and water-soluble chemicals. When a hydrogel lens comes into contact with a drug-containing solution, there will typically be concentration of the drug within the lens. The degree of concentration depends upon a number of factors, such as the water content of the lens, lens thickness, drug concentration, the molecular weight of the drug and the length of time that the lens is in contact (or even presoaked) with the drug.
This natural reservoir effect of gradual drug release over time is often advantageous in that it allows for prolonging therapeutic drug contact and thus better drug penetration. An additional advantage of hydrogel lenses as drug delivery devices is that the therapeutic agent is delivered in a steadier dosing over time. (In contrast, when a topical drop is applied to an eye not wearing a soft lens, some of it may splash or spill out of the eye, and what does stay in often leads to initial relative overdosing, followed by long stretches of relative underdosing. This is compounded by the dilution effect of the tears and blinking.) This reservoir concept has been successfully applied in the use of hydrogel drug delivery with pilocarpine, numerous antibiotics, EDTA for alklai burns, topical steroids, hyperosmotics and lubricating solutions.
Important variables can influence the degree of drug penetration with hydrogel lenses. First, the water content of the lens is perhaps one of the most important, as lenses with higher water content absorb more water-soluble drug compounds for later release into the tear film than do low water contact lenses. Thus, for the purpose of extended drug release, a high water content lens would be the lens of choice. In addition, drug delivery through a high water hydrogel often allows therapeutic levels of the drug to be achieved with lower concentrations of the drug than would have been otherwise necessary in the absence of the contact lens.
Second, the molecular weight of a drug influences its penetration potential into the hydrogel lens. In general, drugs with molecular weights below 500 will be able to penetrate and concentrate within the soft lens matrix. This includes almost all topical eyedrop medications used in everyday practice. High molecular weight fluorescein, in contrast, does not generally penetrate hydrogel lenses, and it can be used when staining a hydrogel lens wearer without fear of contact lens discoloration.
A third important variable is lens thickness. A very thin lens will permit a greater amount of drug to pass into (and out of) the lens, into the post-lens tear film. What this means is that thin lenses do not store drugs efficiently for later time-release, and they are not much better than when an eyedrop is instilled into a non-lens wearing eye. A thin, low water content lens (such as a low water disposable lens) may be an excellent choice as a bandage lens for promoting healing in an epitheliopathy, whereas the same lens would be ineffective in providing higher levels of antibiotic to an infected eye. (For this purpose, a high water, thick lens would be preferred.)
To maximize drug delivery with hydrogel lenses, the clinician should also consider the benefits of presoaking the lens. This helps to equilibrate the lens and provide a more sustained and higher degree of drug release. This concept is often employed in the use of antibiotics for severe ocular infections, or in the use of antibiotics and/or NSAIDs in a hydrogel lens used post-operatively.
How do these aspects of pharmacokinetics apply to preservatives used in contact lens lubricants, salines and disinfection solutions, as well as in topical therapeutic agents? It has long been known that prolonged exposure to solution preservatives can lead to a higher than normal degree of toxic effects, leading to ocular irritation, hyperemia and superficial punctate keratitis (SPK). This has certainly been true in the case of the preservatives benzalkonium chloride (BAK), chlorobutanol and thimerosal. The clinician can observe the effects of classical toxicity most vividly when a soft contact lens is accidentally rinsed or soaked in irrigating solution containing BAK rather than with saline. The patient receiving such a lens will, in a few seconds to minutes, develop a red, irritable eye, upon which lens removal will demonstrate numerous superficial punctate stains across the cornea.
Yet a patient wearing a hydrogel lens who puts a drop of medication containing BAK as a preservative will generally show no such reaction. This is because in the first instance, the lens was thoroughly soaked in, absorbed and even concentrated the BAK, and continued to release the preservative across the entire surface area of the cornea and limbus. The instillation of one drop of medication containing BAK is not enough to significantly concentrate, and is also partially diluted on the lens surface by the tear film and the blink. Using topical ophthalmic medications containing BAK with hydrogel lenses, although an off-label practice, is acceptable as a clinical management decision, as long as lenses are not soaked in these products.
Conclusion
There is a complex relationship between contact lens wear and the effects of medications. This applies to both systemic and topical medications. Practitioners need to be aware of how therapeutic agents can influence successful contact lens wear.
Dr. Silbert is a Professor of Optometry and Director of the Cornea & Specialty Contact Lens Service of The Eye Institute at the Pennsylvania College of Optometry.
References are available upon request. To receive references via fax, call 800-239-4684 and request document #82. Have your fax number ready.