SILICONE HYDROGEL STUDY
Silicone Hydrogel
Lenses in Practice
This study evaluated a silicone hydrogel lens worn by patients with various wearing schedules and lens care systems.
By Michael D. DePaolis, OD, FAAO, and Robert A. Ryan, OD,
FAAO
Silicone hydrogel materials were first made available in the U.S. market in early 1999 when Bausch & Lomb introduced balafilcon A in its PureVision silicone hydrogel contact lenses. The recent FDA approval of silicone hydrogel lenses for up to 30 days of continuous wear has renewed practitioner interest in long-term continuous wear.
Silicone hydrogel lenses have been widely recognized as a technological breakthrough for continuous wear contact lenses. As clinical investigators for continuous wear with silicone hydrogel lenses, we developed an appreciation for the benefits that these new lenses bring to patients seeking freedom from eyeglasses. Because of our early exposure to silicone hydrogel contact lenses and their broad availability since 1999, we have fitted over 200 patients in this new lens material for a wide range of wearing schedules.
This study evaluated the performance characteristics of Bausch & Lomb's PureVision (balafilcon A) silicone hydrogel lens when worn by patients with various wearing schedules and lens care systems.
Methods
We randomly selected 65 files from our patient base of contact lens wearers who had been dispensed PureVision contact lenses.
We included in the study only patients who had worn their contact lenses to the follow-up evaluation and had a complete slit lamp examination. We placed no restriction on the wear schedule or type of lens care system that patients used.
We collected wear schedule, lens care system used, symptoms and slit lamp examination results for each patient.
Results
Wearing Schedules Some 22 patients (33.8 percent) were wearing the PureVision lenses on a daily wear basis. Another 14 patients (21.5 percent) were using the lenses on a 30-day continuous wear schedule, and 29 patients (44.6 percent) were using them on a more traditional seven-day continuous wear or "flex wear" schedule. Two patients were wearing their PureVision lenses monocularly, and for those patients we analyzed only the PureVision-wearing eye. Powers prescribed for all study patients ranged from 9.00D to +5.50D.
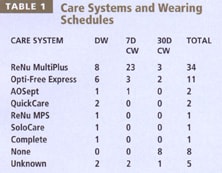
Slit Lamp Findings Table 2 summarizes the slit lamp reports for the 128 eyes evaluated, with grades for each finding assigned as follows: 0 = None, 1 = Trace, 2 = Mild, 3 = Moderate, 4 = Severe. Of the 128 eyes evaluated, only three eyes (2.3 percent) of two patients had a Grade 2 or higher finding during the slit lamp examination. One patient had pre-existing Grade 2 (mild) tarsal conjunctival abnormalities OU. The patient had been wearing PureVision lenses for a year and was currently on a daily wear schedule and caring for the lenses with CIBA Vision's SoloCare. One patient had Grade 2 (mild) corneal staining in one eye. This patient had been using PureVision lenses for four weeks on a daily wear schedule and caring for them with the QuickCare system (CIBA Vision).
Patient Symptoms and Complaints Four of the 65 patients reported some level of symptoms. Two of the patients had been previously fitted with punctal plugs and reported symptoms of discomfort. One of these two patients also complained of redness, burning and discharge. The single patient with a Grade 1 corneal infiltrate had symptoms of discomfort and discharge. A fourth patient reported idiopathic discomfort. There were no eyes with symptoms of dryness.
Discussion & Conclusions
Although silicone hydrogel lenses have been available for continuous wear for more than three years, market research indicates that practitioners dispense lenses for continuous wear for less than 10 percent of new soft lens fits and refits. Silicone hydrogel lenses are prescribed in a variety of modalities, and market surveys indicate that approximately 60 percent of PureVision lens patients are on a daily wear schedule. Therefore, it is important to understand the relationship, if any, between wear schedules and patient complications.
This retrospective evaluation demonstrated that a significant percentage of our patient population has been using silicone hydrogel contact lenses on a daily wear basis without incident. While silicone hydrogel lenses are not being marketed to practitioners for this primary purpose, our results indicate that the lenses can sustain the rigors of daily care and handling. In addition, we found no apparent differences with the two most popular lens care systems, ReNu MultiPlus Multi-Purpose Solution or Opti-Free Express solution.
While this study was not designed to compare the performance of the PureVision lenses vs. patients' previous contact lenses or conventional hydrogel lenses in general, we observed that the silicone hydrogel lenses succeed on several levels. We've noticed less injection and reduced neovascularization. Many patients commented on how much whiter their eyes look. Most patients report that they feel less dryness. The silicone hydrogel lenses also seem to be more comfortable at the end of the day. These benefits appear to apply to both daily and continuous wear patients.
We currently offer 30-day continuous wear to approximately 35 percent of new and existing contact lens patients. Growing patient interest from individuals seeking greater convenience, enhanced contact lens performance, an alternative to laser vision correction or with specific vocational or avocational needs has accentuated this trend. Of course, we endorse continuous wear only after a thorough history and examination to ensure there are no contraindications to this modality.

All of these data, and our own observations, suggest that the PureVision, balafilcon A, lens is a safe and viable technology suitable for daily, seven-day and 30-day modalities.
See Page 3 of the September 2002 Contact Lens Spectrum for details on PureVision in the United States.
Dr. DePaolis is in private group practice in Rochester, New York. He serves as an adjunct faculty member at the Pennsylvania College of Optometry and is a Clinical Associate at the University of Rochester School of Medicine.
Dr. Ryan is in private group practice in Rochester, New York. He is an Associate in Ophthalmology at the University of Rochester School of Medicine.



