MPS OBSERVATION
Views on MPS
With Daily Wear of Silicone Hydrogels
Three clinical researchers share
observations of a clinical finding after using an MPS with daily wear
of silicone hydrogel lenses.
Every year or two an unpredictable observation is made by a perceptive clinician which catches the field by surprise. It is our responsibility to publish these observations in the best interest of your patients and to cover the entire field of contact lens care. Sometimes these observations are controversial and varying degrees of confirmation or refuting observations are available. In the interest of fair balance and presenting various points of view, we offer the following three reports:
- "SPK with Daily Wear of Silicone Hydrogel Lenses and MPS"
- "Observations of Corneal Staining with MPS and Silicone Hydrogel Lenses"
- "Contact Lens and Solution Manufacturer Responds with Data"
Over the years, a number of predictable and unpredictable, as well as subtle, contact lens and solutions interactions have been observed. Group IV lenses and the now obsolete OptiSoak, CSI and Silsoft with Hydrocare disinfecting solutions, for example, could cause mild to moderate superficial punctate keratitis. Used Group IV lenses and sorbic acid solutions cause lens yellowing. The observations reported here fall into this category of lens and solution interactions. One should always consider that there are many variables associated with these observations lens age and deposits, time of soaking, time of wear, individual variations in ocular surface physiology and amount of fluorescein, just to mention a few.
Silicone hydrogel lenses are available throughout the world. They are primarily used for continuous wear, but they are also used for daily wear as problem solvers, lenses of choice and bandage lenses. They are a truly remarkable scientific breakthrough.
Controlled studies often result in very clean data. Out in the "real world," other things can happen. Is that the outcome here? If a finding is for real, it would be reported by others. Please stay tuned to Contact Lens Spectrum for more on the significance of this finding and possibly other reports.
Fortunately for both patient and practitioner, many options are available today, both in contact lenses and contact lens care.
Joseph T. Barr, OD, MS, FAAO, Editor

Director of Contact Lens Service, North Shore University Hospital, Great Neck, NY Private contact lens specialty practice |
|
SPK with Daily Wear of Silicone Hydrogel Lenses and MPS
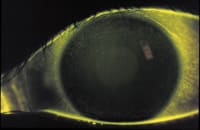
Several months ago, I encountered an unreported clinical finding. One of my previously successful patients returned for a scheduled routine follow-up visit. The patient had no significant complaints or symptoms, but during a routine slit lamp examination, a coarse dense diffuse superficial punctate staining was noted (Figure 1). This patient had worn PureVision (Bausch & Lomb) silicone hydrogel lenses successfully for over a year on a weekly extended wear schedule. Previous examinations had been unremarkable. I asked the patient to identify any changes in her health status, environment or in the use and care of her lenses. Only two things had changed:
- The patient had discontinued weekly extended wear and was now wearing and caring for the lenses on a daily wear schedule.
- The patient had switched from Alcon's Opti-Free Express Multi-Purpose Disinfecting Solution to Bausch and Lomb's ReNu Multi-Plus Multi-Purpose Solution.
This observation reminded me of reports that appeared some years ago about the untoward combination of Silsoft or CSI (CIBA Vision) lenses with the now obsolete HydroCare solution (Allergan) or the reactions of Group IV lenses with Opti-Soft (Alcon's precursor to Opti-Free). The puzzling aspect of the current observation was that the staining occurred in the absence of significant patient complaints of discomfort.
To help pinpoint possible causes of this complication in daily wear patients using silicone hydrogel lenses, I first looked at patients in my own practice already on this wearing schedule. All were using Opti-Free Express. I found no significant staining or other remarkable findings. To expand this evaluation I placed seven volunteers from my practice, four wearing silicone hydrogels and three wearing traditional hydrogel materials, on a daily wear schedule using PureVision silicone hydrogel lenses with ReNu Multi-Plus solution following the rub and rinse directions on the label.
|

|
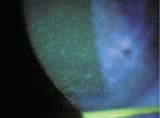
| Figure 1. Dense SPK in a patient wearing PureVision lenses on a daily wear basis and using ReNu MultiPlus | Figure2. Study patients developed SPK after two weeks. |
Initially all patients were asymptomatic and had no significant corneal findings. After one week, all patients showed either minor clinically insignificant SPK or no corneal findings. After two weeks, one patient who showed negligible findings complained of significant lens awareness and discomfort. Three other patients exhibited SPK on slit lamp examination. One case was relatively mild, but the two others had dense coarse superficial punctate keratitis of both corneas, not unlike the initial patient who had presented earlier at my practice (Figure 2). Due to the severity of these findings, I discontinued the study early, all eyes resolved without sequellae and patients returned to their original wear schedule, lenses and care products.
This unexpected observation of superficial epitheliopathy in wearers of silicone hydrogel lenses using ReNu Multi-Plus remains unexplained. With the positive attributes of these new-generation lenses, the ability to freely use them under a variety of wearing schedules and conditions is important. In addition, a significant number of practitioners and patients may already be using these state-of-the-art lenses on a daily wear basis. Since these findings remain largely anecdotal, I believe that it is important for a rigorous scientific investigation to be conducted to replicate and explain these observations. Pending this study, practitioners should carefully monitor patients wearing silicone hydrogel lenses who use PHMB- based care products.

Director, Centre for Contact Lens
Research |
|
Observations of Corneal Staining with MPS and Silicone Hydrogel Lenses
Corneal staining occurs with all soft contact lenses. Nichols et al reported an incidence of 8 percent for moderate/severe staining from a multi-center study. While it can be difficult to establish the etiology, diffuse punctate epitheliopathy that stains with fluorescein is often associated with solution sensitivity or toxicity, depending on the severity. It has been shown that increased levels of staining occur with polyaminopropyl biguanide (PHMB)-based systems compared with Polyquad or hydrogen peroxide-based regimens. A study conducted at the Centre for Contact Lens Research at the University of Waterloo, comparing Bausch & Lomb's Optima FW and PureVision lenses using ReNu Multiplus disinfecting solution revealed equal levels of corneal staining with each lens (unpublished data). Jones et al reported significant levels of corneal staining with N-vinyl pyrrolidone (NVP)-containing Group II material (alphafilcon; Bausch and Lomb) when used with a solution containing high levels of PHMB.
Our experience of corneal staining with daily wear of PureVision lenses when disinfected with ReNu MultiPlus solution is different to that stated and shown by Dr. Epstein. When we have observed the staining pattern, it was invariably asymmetric, where the center of the cornea is invariably spared and the severity increases towards the periphery resulting in circumferential staining as shown in Figure 1. Although the staining is superficial, it is fairly dense.
|
|
|
|
Figure 1. Circumferential staining with a clear cornea. |
Dr. Epstein reports challenging seven patients with PureVision lenses and ReNu Multiplus solution. At one week the patients who were examined had either no or clinically insignificant SPK, and this is invariably what we have found. Of the four patients who were examined at two weeks, one had mild SPK and two others had coarse and dense SPK covering the entire cornea which necessitated interruption of the study. The corneal staining of these two patients resolved without sequellae, which is consistent with what we have observed. The staining patterns that we have observed are superficial and clear rapidly upon cessation of lens wear.
Another important feature of patients using ReNu MultiPlus with PureVision lenses is that they are usually asymptomatic except for some who have a burning/stinging sensation on lens insertion. However, patients who experience this irritation on insertion are not necessarily the ones who exhibit corneal staining, as corroborated by Dr. Epstein. As it is likely that the staining would dissipate without cessation of lens wear, it may explain why patients are asymptomatic except immediately after lens insertion.
Managing patients who develop corneal staining when using ReNu MultiPlus with PureVision is simple. Although patients may be asymptomatic, staining can be eliminated by using another solution, such as one containing a lower concentration of PHMB, Polyquad-based or hydrogen peroxide systems. Another option is patients rinsing and rubbing their lenses with preserved saline solution prior to insertion.
Silicone hydrogel contact lenses have been on the international market for about three years, and according to manufacturers reports, approximately 500,000 patients are wearing them. About 40 percent of these patients are wearing the lenses on a daily wear basis. On the assumption that there are 100,000 daily wearers of PureVision lenses, the majority would be using PHMB-based solutions. Yet to the best of my knowledge, Dr. Epstein's report is the first of its kind. One possible reason is that patients are asymptomatic and not returning for follow-up visits. Another is that practitioners are unconcerned about the level of staining and are managing the condition as described above or are not observing this type of reaction.
To receive references via fax, call (800) 239-4684 and request document #87. (Have a fax number ready.)

Vice President Medical and Clinical Affairs, Bausch & Lomb Incorporated, Rochester, NY |
|
Contact Lens and Solution Manufacturer Responds with Data
We take seriously the anecdotal reports by Dr. Epstein about his three patients, and as a result have undertaken a review of our clinical data. Neither the clinical data, nor the surveillance data at our disposal, support the findings reported in this paper.
Over the past several years, Bausch & Lomb has conducted numerous evaluations that included PureVision contact lenses and ReNu MultiPlus Solution and ReNu Multi-Purpose Solution. To avoid errors of chance occurrence, patient sample sizes were defined by statistical modeling to detect differences in performance among normal populations. To avoid practitioner and geographical location bias, these evaluations included multiple investigators and locations. To provide greater scientific validity, appropriate controls, such as conventional hydrogel lenses, were used as a basis for comparison. Collectively, there have been several thousand contact lens patients and over 100 independent practitioners who have participated in these investigations. Levels of corneal staining in these studies were no different than the conventional hydrogel controls.
More specific to Dr Epstein's observations, two prospective, multi-centered studies were conducted in which PureVision lenses were worn by patients in a daily wear modality and ReNu MultiPlus was used as the lens care system. Eighteen investigators enrolled a total of 381 patients. There were nearly 800 visits examining the performance characteristics of the products in these evaluations. Table 1 contains a summary of slit lamp findings obtained from these studies. The analysis of corneal staining indicated that 3.3 percent of the observations were graded as mild or greater for the eyes wearing PureVision lenses. For the eyes wearing lenses of conventional hydrogel materials, two percent of the observations were for corneal staining graded as mild or greater. This difference was not statistically significant. However, it was noted that there was significantly less limbal and bulbar injection in patients using PureVision and ReNu MultiPlus versus the conventional hydrogel/ReNu MultiPlus control.
In addition to the scientific clinical evaluation of our lens and lens care products, Bausch & Lomb routinely monitors post-market product performance data. A review of this data for the entire year of 2001, including all possible complaints, yielded complaint rates of 0.003 percent for ReNu MultiPlus and 0.01 percent for PureVision lenses.
As a responsible company, Bausch & Lomb continuously monitors and evaluates the post-market performance of all of our products. Our evaluations clearly support the continued combined use of PureVision lenses and ReNu MultiPlus Multi-Purpose solution.