CONTACT LENS MATERIALS
Modern Contact Lens Materials: A Clinical Performance Update
This comprehensive look at contact lens
materials examines oxygen requirements of the cornea and broad differences among materials.
By Lyndon Jones, PhD, FCOptom, FAAO
Every day, practitioners question what contact lens type to choose. Options increase monthly, with each new material reportedly offering advantages over its predecessors or competitors. Properties of an "ideal" contact lens material are detailed in Table 1.
A basic understanding of the materials clinicians use on a daily basis is critical to understanding many of the clinical observations seen during their use. Subtle differences in the chemical composition of materials can impact a wide variety of clinical factors, including oxygen transport, dehydration, parameter stability and deposition.
|
|
|
|
Figure 1. Dk versus water content for conventional and silicone hydrogel materials. |
|
Corneal Physiology and Oxygen
The cornea is avascular and derives most of its oxygen from the atmosphere. A sufficient oxygen supply to the cornea maintains corneal integrity and provides defense against infection. Any contact lens acts as a potential barrier to oxygen transport to the anterior ocular surface, and the ability of a material to transport oxygen through the lens is a major factor in determining the clinical success of that material. The average level of oxygenation required at the anterior cornea to prevent edematous complications is 10 percent EOP (equivalent oxygen percentage) for daily wear and 18 percent EOP for closed eye conditions (Holden 1984, Brennan 1988), although individual variations are marked (Efron 1986) and age differences do arise (Polse 1989).
To determine if contact lenses provide patients with such levels of oxygen, it is necessary to examine how easily oxygen transfers through the lens material. To establish this, lens oxygen transmissibility (Dk/t), which takes into consideration both material oxygen permeability (Dk) and lens thickness (t), must be evaluated. The units of Dk are 10-11 (cm2/ sec)(mlO2/ml x mmHg) or "barrer," and the units of oxygen transmissibility are 10-9 (cm/sec)(mlO2/ml x mmHg) or barrer/cm.
Traditional soft lens materials rely on water to transport oxygen through the lens, as oxygen dissolves into the water-phase of the material and diffuses through the lens from the anterior to the posterior lens surface. This has been a limiting factor, because water has a Dk value of only 80 barrer (Fatt 1986). The Dk of conventional hydrogels increases logarithmically with the equilibrium water content of the material (Ng 1976). To determine the Dk of conventional soft lenses, the non-edge corrected Fatt formula (Dk = 2.0 x 10-11 e0.0411WC) (Fatt 1982) or boundary and edge-corrected Morgan and Efron formula (Dk = 1.67 x 10-11 e0.0397WC) (Morgan 1998) can be used, in which "WC" is the quoted water content of the material concerned. Once the Dk of the material is known, then the clinician determines the Dk/t of the lens by assessing lens thickness (Fatt 1993, 1994). By contrast, gas permeable (GP) lenses transmit oxygen via the polymer phase, with no oxygen diffusion occurring through the water phase.
|
|
TABLE 1: Properties of an Ideal Contact Lens Material |
|
Meet or exceed the cornea's oxygen requirements Be physiologically inert Provide excellent in-eye wetting Resist deposition Provide dimensional stability Provide reasonable durability Be optically transparent Require minimal patient care Provide easy and cost-effective manufacture |
To establish if a lens will provide sufficient oxygen, determine the amount of oxygen required by the patient's cornea. The most widely cited figures for the minimum acceptable Dk/t are 24 x 10-9 barrer/cm for daily wear and 87 x 10-9 barrer/cm for overnight or extended wear (Holden 1984). Recently, a level of 125 x 10-9 barrer/cm has been reported as a requirement to prevent stromal anoxia during closed-eye conditions (Harvitt 1999). These values are "averages," and patients exhibit different corneal metabolic requirements (Efron 1986, Larke 1981).
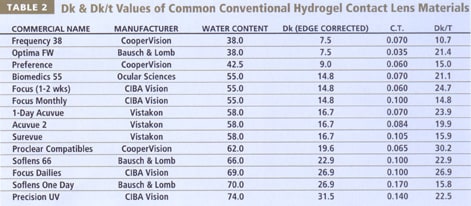
The use of Dk/t values by companies to describe differences among materials may be misleading, due to a lack of standardization. Materials are often quoted as having high Dk values, and the thickness values used are usually quoted at a given center thickness on a 3.00D lens. This provides little useful information about individual lens performance, particularly with hypermetropic and high minus lenses (Holden 1983, Bonanno 1986, La Hood 1991). High-water content soft lenses need to be made thicker than their low-water counterparts to prevent pervaporation (Holden 1986, McNally 1986) and facilitate ease of handling, resulting in relatively low Dk/t values compared with their considerably higher Dk values. Consequently, thicker high-water content soft lenses and ultrathin low-water content soft lenses deliver about the same amount of oxygen to the central cornea in a minus prescription (Table 2), with moderate advantages for the high water-content lenses in the peripheral zones (Jones 1991).
The close relationship between water content and oxygen permeability for conventional soft lenses has impeded hydrogel lens material development for extended wear for more than 20 years. Table 2 shows that conventional lens materials provide inadequate oxygen transmissibilities for safe, edema-free overnight wear.
Contact Lens Material Composition
Lens materials are either water-containing soft (hydrogel) or non-water containing GP materials. Over 90 percent of patients wear hydrogels (Morgan 2002, Barr 1997), due to their increased initial comfort and reduced sensation of dryness. Within hydrogel lenses two subcategories exist: conventional and silicone hydrogel lenses.
All contact lens materials are polymers, which are either "homopolymers" that contain multiple repeat units of the same monomer, or "copolymers," which are composed of more than one monomer type joined together. The constituent monomers which comprise the polymers determine the physical and chemical properties of the materials. Repeating chains of monomers are arranged in patterns with cross-linking between the polymer chains to afford strength and further govern the characteristics of the lens materials, with ethylene glycol dimethacrylate (EGDMA) a commonly employed cross-linker in soft lens materials (White (1994, Tighe 2002).
|
|
|
|
Figure 2. Atomic force microscopy pictures of
etafilcon, balafilcon and lotrafilcon surfaces. The lathe marks on the surface of the etafilcon lenses (A) are visible. The "island" appearance of the balafilcon surface (B) and the homogenous surface of the lotrafilcon material (C) are
different. |
|
Rigid Lens Materials
Perspex or polymethylmethacrylate (PMMA) lenses were first fitted in the 1940s and remained the standard for lens wear until the commercialization of hydrogel lenses in the early 1970s. While it remains an excellent material in terms of cost, stability, wettability and manufacturing ease, PMMA's inability to transmit oxygen obviously limits its practical use. The development of modern-day GP materials from PMMA is best understood through a full review of the patent literature (Kishi 1988, Tighe 1997).
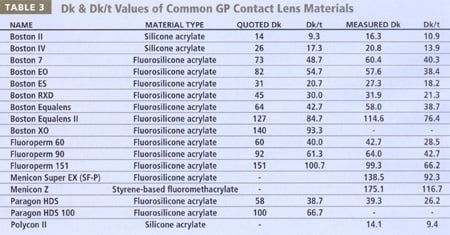
The work of Norman Gaylord and others in the mid-1970s resulted in the development of rigid lenses which incorporated either silicon (siloxymethacrylates) or fluorine (fluorocarbon methacrylates), significantly enhancing the oxygen transmissibility of rigid lenses above that of both PMMA and conventional hydrogel materials. Since the introduction of GP lenses into clinical practice in the early-to-mid 1980s, manufacturers have adjusted the ratios of methyl methacrylate, silicon, fluorine and various wetting agents to provide the best possible balance of oxygen permeability, wettability, dimensional stability and deposit resistance (Tighe 2002). Table 3 presents a list of several common GP materials and their recently reported Dk values (Benjamin 2002), which are frequently lower than that reported by the manufacturer (RGPLI 2001). Table 3 provides both the edge and boundary corrected values (Benjamin 2002) and non-compensated values. For comparative purposes with soft contact lenses presented earlier (Table 2), Dk/t is also presented for both Dk values, at a common center thickness of 0.15mm.
Hydrogel Lens Materials
Hydrogels are water-absorbing, hydrophilic polymeric materials that are plasticized by the water they absorb and have been used in implant devices within the human body (Pedley 1980, Corkhill 1990), with the first successful hydrogel material (poly-2-hydroxyethyl methacrylate or polyHEMA) developed by Otto Wichterle in the late 1960s as a general-purpose surgical material (Wichterle 1960). In the ocular arena, hydrogels are utilized as intraocular crystalline lenses, scleral buckles, drug delivery devices and, most commonly, contact lenses.
Conventional Hydrogel Materials
Conventional hydrogel lens materials consist of a "backbone" material that provides parameter stability. The amount of water absorbed by the hydrogel is described by the term "equilibrium water content" (EWC), and this factor strongly influences the final polymer's surface, mechanical and transport properties. The principal backbone material used is polyHEMA, which, as a homopolymer, has a water content of 38 percent. Various monomers are added to provide variations in wettability and oxygen transportation. Two principal strategies have historically been employed to increase the water content of hydrogels above that of polyHEMA:
- Small quantities of negatively charged groups such as methacrylic acid (MA) or
- Larger amounts of more hydrophilic, neutral groups such as polyvinyl alcohol (PVA) or N-vinyl pyrrolidone (NVP) are added to raise the water contents to 60 percent or greater (White 1994, Tighe 2002).
Addition of hydrophilic monomers such as NVP or MA increases water content but reduces physical strength. Addition of relatively hydrophobic monomers such as methyl methacrylate (MMA) reduces water content but increases the mechanical characteristics of the materials (Tighe 2002).
Silicone Hydrogel Materials
The recent release of a new family of hydrogel materials based on silicone technology (termed silicone hydrogels) has introduced a new generation of lenses. They are intended for continuous in-eye wear for up to 30 days, and the term "continuous wear" has become synonymous with their use (Sweeney 2000).
Silicon-containing flexible lenses are not new; silicone-elastomeric lenses have been used for therapeutic and pediatric applications for many years (Gurland 1979). These lenses offer exceptional oxygen transmission and durability, but limitations are associated with their use in clinical practice. Fluid is unable to flow through these lens materials, resulting in frequent lens binding to the ocular surface (Rae 1991), and the lens surfaces are hydrophobic, resulting in marked lipid deposition (Huth 1981).
In silicone hydrogel materials, silicone rubber is combined with conventional hydrogel monomers. The silicone component provides extremely high oxygen permeability, while the hydrogel component facilitates fluid transport and lens movement. This combination was a formidable challenge, and it has taken considerable time to create these materials and designs. The process of combining these monomers has been likened to combining oil with water, while maintaining optical clarity (Tighe 2000). Explanations of the development of silicone hydrogel materials have been described previously (Tighe 2000, Kunzler 1993, Nicolson 2001, Friends 1995, Kunzler 1999) and this description provides a brief overview.
Currently, two silicone hydrogel lenses are available. A novel silicone hydrogel coated with phosphorylcholine recently described (Court 2001) is not yet available. Table 4 summarizes the differences between the two silicone hydrogel materials and compares them with Vistakon's Acuvue material. Contact lens materials must permit both oxygen and ion transmission. One approach to achieve this goal incorporates of two "phases" into the materials. Phase separation occurs when the interconnections between the chemically similar molecules in the material are stronger than the adhesive connections between them and the different molecules. This approach was usually avoided because it resulted in an opaque material. Techniques have been developed in which the phase separation is limited, such that the phase size is far shorter than the wavelength of light, resulting in optically clear materials (Nicolson 2001).
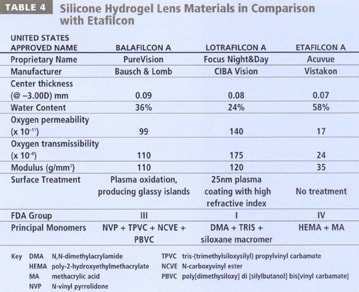
CIBA Vision's Focus Night & Day material, lotrafilcon A, employs such a biphasic or two-channel molecular structure (Nicolson 2001). The fluorosiloxane phase facilitates storage and transmission of oxygen, and the hydrogel phase transmits water and oxygen, allowing good lens movement. The two phases work concurrently to allow the co-continuous transmission of oxygen and ions. Lotrafilcon A is comprised of a fluoroether macromer co-polymerized with the monomer trimethylsiloxy silane (TRIS - used in the preparation of GP materials) and N, N-dimethyl acrylamide (DMA), in the presence of a diluent (Lopez-Alemany 2002). The resultant silicone hydrogel material has a water content of 24 percent and an oxygen permeability (Dk) of 140 barrers (Alvord 1998). Bausch & Lomb's PureVision material, balafilcon A, is a homogeneous combination of the silicone-containing monomer polydimethylsiloxane (a vinyl carbamate derivative of TRIS) co-polymerized with the hydrophilic hydrogel monomer N-vinyl pyrrolidone (NVP) (Tighe 2002, Kunzler 1999, Lopez-Alemany 2002, Grobe 1999). This silicone hydrogel material has a water content of 36 percent and a Dk of 110 barrers.
Novel silicone hydrogel lenses have unique properties due to the fundamental differences between these materials and conventional hydrogels. These differences include oxygen transmission, ion transport, rigidity and surface wettability.
Oxygen Transmissibility
In silicone hydrogel materials, oxygen is transmitted primarily through the silicone component of the material, resulting in a dramatic increase in the oxygen permeability, as compared with conventional hydrogels. Pure silicone rubber has a Dk of 400 to 600 barrers, and this provides silicone hydrogel materials with Dk/t values of 110 to 175, six times more permeable than conventional hydrogel materials.
Figure 1 is redrawn from Tighe (Tighe 2000) and demonstrates that the Dk of silicone hydrogel lens materials is vastly different compared with conventional lens materials, in which the Dk is directly related to the water content. As a result of the increased oxygen availability, studies conducted at the Centre for Contact Lens Research (CCLR) have reported overnight edema levels with the new generation materials to be similar to levels seen with no lens wear, and far lower than those measured with disposable soft lenses (Fonn 1999). Central corneal swelling induced by an etafilcon A (Acuvue) lens on eye opening was significantly higher than with a lotrafilcon A lens (8.7 ± 2.8 percent vs. 2.7 ± 1.9 percent, p<0.00001). The de-swelling profiles following lens removal were much quicker for the lotrafilcon A-induced corneal swelling (100 minutes) than for etafilcon A-induced swelling, which took almost twice as long to return to baseline levels. In a similar study, the overnight central corneal swelling induced by balafilcon A lenses (PureVision) was found to be 2.8 ± 2.0 percent compared to 8.7 ± 2.7 percent with a 70 percent water content lens (Fonn 2002).
In addition to a reduction in acute signs of hypoxia such as corneal swelling, studies have shown that the use of silicone hydrogel contact lenses for overnight wear has resulted in a significant reduction in the chronic signs of hypoxia, including limbal hyperemia, microcysts and myopic progression (Dumbleton 2001, du Toit 2001, Covey 2001, Sweeney 2000, Keay 2000, Dumbleton 1999, Papas 1998, Papas 1997, Morgan 2002, Brennan 2002). Results from a plethora of studies now indicate that the increased level of oxygen afforded to corneas under silicone hydrogel lenses has resulted in no (or at worst minimal) signs of hypoxia, either chronic or acute, following their overnight use.
|
|
|
|
Figure 3. Lipid deposition in the form of lens calculi (jelly bumps) on a silicone hydrogel lens. Image courtesy of Brian
Tompkins |
Fluid and Ion Permeability
Transporting fluid and ions through contact lenses is crucial for the provision of essential nutrients and removal of waste products and debris. Water flow through the lens is also necessary for on-eye lens movement, comfort and wettability (Nicolson 2001). The hydrogel component of lens materials is responsible for these processes. In homogenous silicone hydrogel materials such as balafilcon A, while the oxygen permeability increases, the hydraulic permeability decreases with decreasing water content. This is because fluids and ions are transported through the hydrogel component of the material. A minimum sodium ion and hydraulic permeability of 0.2 x 10-6 cm2sec-1 is required for adequate lens movement (Tighe 2000). A balance has to be reached between maximizing oxygen transmission while still allowing sufficient hydraulic flow to prevent hydrophobic binding of the lens to the cornea.
In biphasic co-continuous silicone hydrogel materials such as lotrafilcon A, the oxygen and fluid permeability are "uncoupled," allowing a much greater level of hydraulic and ionic permeability than would be available through a polyHEMA-based material with an equivalent water content. Lenses made from this material display adequate movement while still benefiting from the additional oxygen permeability afforded with a 24 percent water content. In the case of the balafilcon A material, a 36 percent water content provides a hydraulic permeability which corresponds with that normally offered by a 40 percent water content lens. This suggests that there may also be some degree of phase separation of the material.
Plasma Surfacing Treatments
Historically, a huge impediment to the development of silicone hydrogel lenses has related to the decreased wettability, increased lipid interaction and accentuated lens binding inherent in silicon-based materials, as previously described. In order to render the surfaces hydrophilic, techniques incorporating plasma into the surface processing of the lens have been developed (Grobe 1999).
The surfaces of Focus Night & Day contact lenses are permanently modified in a gas plasma reactive chamber to create a permanent, ultrathin (25nm), high refractive index, continuous hydrophilic surface (Nicolson 2001, Weikart 2001). PureVision lenses are surface-treated in a gas plasma reactive chamber which transforms the silicone components on the surface of the lenses into hydrophilic silicate compounds (Tighe 2002, Tighe 2000, Lopez-Alemany 2002, Grobe 1999). Glassy, island-like, discontinuous silicate "islands" result (Lopez-Alemany 2002), and the hydrophilicity of these areas "bridges" over the underlying hydrophobic balafilcon A material. Using very high magnification imaging techniques such as atomic force microscopy (AFM), the subtle differences in the surfaces of these materials can be appreciated (Figure 2). These surface modifications do not impede the flow of oxygen and fluids through the lenses. Both surface treatments become an integral part of the lens and are not surface coatings that can be "stripped" away from the base material.
Silicone-elastomeric materials are extremely elastic and tend to adhere to the cornea more readily. The material elasticity of silicone hydrogel lenses is much less and fortunately approaches that of HEMA. This further helps to prevent lens adhesion and promote movement and tear flow beneath the lens. Silicone hydrogel lenses are much "stiffer" than their conventional hydrogel counterparts, due to the incorporation of silicon. The modulus of the materials is 110 to 120 g/mm2 (1.1 to 1.2 MPa) (Tighe 2000) which is over twice that of polyHEMA and nearly four times greater than the etafilcon A material (Acuvue).
Increased rigidity or stiffness has some advantages, in that the lenses handle very well and are a perfect choice for people who exhibit poor handling capabilities. Increased rigidity might also suggest that such lenses may mask more corneal astigmatism than traditionally very flexible hydrogel lenses, but that has not been our experience clinically. The mechanical properties of these lenses do pose some problems in that they are less able to conform easily to the shape of the eye and fitting is critical, with loose lenses exhibiting poor comfort. Additionally, the rigidity of these materials may be implicated in a variety of mechanical complications seen with silicone hydrogel lenses, including papillary conjunctivitis and superior epithelial splits (Holden 2001, Jalbert 2001, O'Hare 2001, Skotnisky 2002, Jones 2002, Dumbleton 2002).
Hydrogel Classification Systems
With such a wide variety of soft contact lens materials available to clinicians, it is important that some form of classification system exists to delineate materials which are different from each other. To complicate matters, four such systems currently exist to subdivide and classify hydrogel materials:
- Commercial name This is the name given to the lens by the manufacturer and is the typical brand name by which practitioners and patients alike know a particular lens type. Examples include Acuvue, Proclear, Soflens 66 and Frequency 55.
- United States Adopted Name (USAN) This is the unique name for a material consisting of a certain fixed mono-mer composition. Examples include etafilcon, tetrafilcon, omafilcon and vifilcon. Polymacon is a generic term for polyHEMA lenses and several commercial names will be given to lenses that have the same USAN. A typical example includes Acuvue, 1-Day Acuvue and Surevue, all of which are etafilcon-based lenses. Some historic anomalies to this system do exist, with the same USAN being assigned to materials of differing water content. Examples include bufilcon and phemfilcon (Tighe 2002).
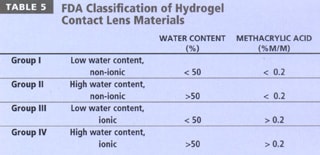
- FDA categorization This is a simple subdivision of lens materials based upon water content and ionic charge (Table 5). Materials with water contents greater than 50 percent are classified as "high water content," and those with greater than 0.2% ionic material (invariably methacrylic acid) are termed "ionic" in nature. This classification is useful to describe the way in which materials interact with both lens solutions and the tear film, as described below.
- ISO classification This system is rarely described and is outlined in greater detail in a number of ISO documents, including BS EN ISO 11539:1999 and BS 7208-2:1991 (BSI 1991, BSI 1999). In this system, rigid lens materials are given a suffix of "focon" and hydrogels a suffix of "filcon," followed by a series of letters and numbers. Examples include Filcon 1a (polyHEMA) and Focon 1a (polymethylmethacrylate) (Kerr 2001).
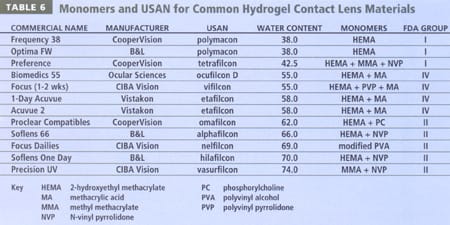
Table 6 details common hydrogel materials and indicates their monomeric composition, in addition to their USAN, FDA grouping and water content. A review of Table 4 will indicate that silicone hydrogel lenses have been classified into FDA groups I and III, although the properties of these materials are not suitably described within the current classification system. A fifth group may be required to adequately describe hydrogel lenses that incorporate silicon.
A basic understanding of the material properties described above is critical to successfully managing several issues that frequently arise in clinical practice, including dehydration, deposition, durability, parameter stability and wettability.
Dehydration
A major problem with contact lenses continues to be their reduction in perceived comfort over the wearing period, particularly as the lens surface dehydrates. Dry eye symptoms are reported by 20 to 50 percent of soft contact lens wearers (Orsborn 1989, Doughty 1997), with 35 percent of patients permanently ceasing lens wear due to complications associated with discomfort and dryness (Weed 1993).
The sensation of "dryness" is a complex subject and is without question related to a variety of factors. One factor to consider is that of lens dehydration, as the subjective symptom of dryness appears to occur more frequently in soft lens wearers whose lenses undergo greater dehydration during open-eye wear (Efron 1988). Potential factors that may explain dehydration-induced discomfort include increased lid-lens interaction through alterations in lens front-surface wettability, alterations in lens fit or the development of epithelial staining due to pervaporation and subsequent desiccation (Holden 1986, Pritchard 1995, Fatt 1980, Orsborn 1998).
All hydrogel lens materials dehydrate during wear (Pritchard 1995, Andrasko 1980, Brennan 1988, Efron 1987, Efron 1999). Dehydration is influenced by a number of factors, including the surrounding environment (Andrasko 1980, Brennan 1988, Efron 1987), water content (Brennan 1988, Brennan 1987), water binding properties (Larsen 1990, Benz 1997), thickness (Andrasko 1983) and wearing period (Wechsler 1983). In addition, significant inter-subject differences exist (Brennan 1988, Efron 1987).
The degree of in vivo material dehydration measured following a period of lens wear varies depending upon the technique used to determine water loss. Polymacon and omafilcon lenses lose approximately 2 percent absolute water content (Pritchard 1995, Efron 1987, Efron 1999, Brennan 1987, Fonn 1999), and etafilcon reportedly loses 6 to 10 percent of its absolute water content (Pritchard 1995, Efron 1999, Brennan 1990, Hall 1999).
The impact of material composition on dehydration is irrevocable, in that materials do dehydrate by differing amounts and at differing rates (Efron 1999). However, the relationship between dehydration and in-eye comfort remains controversial, with some studies finding that increased dehydration results in reductions in lens comfort (Efron 1998, Hall 1999, Young 1997, Lemp 1999) and others finding that no such correlation exists (Pritchard 1995, Fonn 1999).
In a clinical environment, the majority of silicone hydrogel lens wearers of the new report that their lenses feel less "dry" than their previous conventional lenses, despite considerably longer wearing times (Fonn 2000). These novel materials, which have lower water contents than currently available materials, may produce less subjective dryness symptoms through reduced in-eye dehydration, enhanced wettability, reduced hydrophobic interactions with the eye-lid, reduced deposition and/or increased oxygen performance. Published work to date (May 2000) shows that silicone hydrogel lens materials dehydrate at a slower rate and to a lesser extent than conventional hydrogel materials and may help to explain this reduction in the sensation of dryness.
If patients do complain of in-eye dryness, consider moving to another material (such as omafilcon (Hall 1999, Lemp 1999) or a silicone hydrogel) (May 2000), changing to a frequent replacement option and/or using rewetting drops.
Deposition
The deposition of contact lenses with substances derived from the tear fluid is a well-known clinical complication, resulting in reductions in comfort (Pritchard 1996), vision (Gellatly 1988) and increased inflammatory responses (Mondino 1982). Hydrogel materials rapidly spoil with constituents from the tear film, particularly protein (Baines 1990, Bohnert 1988), lipids (Bontempo 1997) and mucin (Castillo 1986). The adsorption of proteins and lipids at the contact lens interface depends on a number of factors, such as material water content (Minarik 1989, Fowler 1985), surface charge (Minarik 1989, Minno 1991, Sack 1987), wearing period (Jones 2000) and age of the lens material (Jones 1996).
Increasing water content and/or ionicity of the lens material greatly enhances protein deposition (Baines 1990, Minarik 1989, Fowler 1985, Sack 1987, Garrett 1998, Garrett 1999, Garrett 2000), with lysozyme being detectable on FDA Group IV lenses after wearing times of as little as one minute (Leahy 1990). While Group IV lenses tend to predominantly deposit lysozyme, neutral Group II lens materials (particularly those containing vinyl pyrrolidone) have a tendency to deposit lipid (Jones 2000, Jones 1997, Maissa 1998). Refitting a patient from a Group IV lens to a Group II lens material may result in the development of excessive lipid deposits. If this occurs, simply knowing the composition of the lens material will explain the sudden appearance of the lipid.
To date, the degree of in-eye biocompatibility achieved with silicone hydrogel materials has received minimal attention (McKenney 1998, Jones 2001, Louie 2000, McNally 2002), and their use within the context of the general biomaterials field remains to be investigated. The only published results to date indicate that the deposition of protein on these materials is less than that seen with conventional materials, but that lipid deposition can be a problem for certain patients (Jones 2001). Figures 3 and 4 reveal various lipid deposition patterns seen in certain patients using silicone hydrogel lenses.
If patients deposit their lenses with lipid, then moving to non-NVP-containing materials (such as Proclear or Acuvue) will reduce lipid deposition. Further options include adding surfactant cleaners containing alcohol (such as Miraflow) or moving to more frequent periods of replacement (Jones 2002).
Durability
One of the major problems with higher water content materials continues to be their increased fragility compared with their low water content counterparts, due to the increased water content separating the polymer chains and reducing their cohesion. The average length of life of a high water content lens is about six months (Jones 1996, Jones 1990), compared with 11 months for low water content lenses (Haig-Brown 1985). Monomer composition similarly impacts on the life expectancy of rigid lenses, with higher Dk RGP lenses lasting a shorter period of time than low Dk lenses (Jones 1996).
One of the driving forces behind the development of frequent replacement systems was the desire for practitioners to move patients into lens materials that provided higher levels of corneal oxygenation, without complications induced by shorter lens life. If practitioners do refit patients with higher Dk materials, then the fact that lenses will last a shorter period of time should be considered. The almost universal adoption of fitting hydrogel lenses on a planned replacement basis has obviated this problem with soft lenses, but relatively small numbers of practitioners fit RGP lenses on this basis, despite the improvement in clinical success when such a system is adopted (Woods 1996). To date, silicone hydrogel lenses are available only as a one-month frequent replacement option, so their length of life on a non-planned replacement basis remains unknown. However, as more silicone hydrogel options become available, this issue may become important.
Parameter Stability
The volume of water absorbed by hydrogels influences the physical parameters of the lenses and can have an adverse effect on lens fit. PolyHEMA is remarkably stable to alterations in the surrounding environment and variations in temperature, pH and tonicity have little effect on the parameters of lenses manufactured from HEMA (Tighe 2002). The addition of monomers to increase water content (whether neutral or charged) can affect this stability. Behavior extremes are found when comparing FDA Groups I and IV materials (Tighe 2002).
Changes in temperature from room temperature to eye temperature results in a drop in water content for all conventional contact lens materials and a corresponding drop in oxygen transmissibility (Efron 1999). This drop is relatively minor for Group I lenses, but it can be clinically significant for Group IV lenses (Efron 1999).
Following soft lens insertion, lens movement will reduce over the first five to 15 minutes of wear (Martin 1983, Brennan 1994). While clinical acumen would surmise that this reduction in lens fit was due to a loss of water from the lens, resulting in a steepening of the base curve and subsequent reduction in movement due to a smaller sagittal lens depth, this point is controversial, with some studies finding that this initial lens tightening is not due to dehydration-induced steepening of the base curve (Golding 1995). One alternative theory to explain what happens is that a reduction in the post-lens tear film occurs following lens insertion, and it is this thinning of the tear film that subsequently reduces contact lens movement on eye (Little 1995, Little 1994).
One final point to consider is the influence of alterations in tonicity and pH brought about through exposure to various care regimens. This is most extreme when considering the influence of peroxide-based systems on FDA Group IV materials. Certain peroxide systems are manufactured with highly acidic pH values close to 4 (Harris 1989). When ionic lens materials are exposed to such low levels of pH, the lens materials shrink due to a loss of water content (McCarey 1982, Harris 1989), which can result in a very tight fitting lens if the lens material is not allowed to neutralize for a sufficiently long period post-disinfection (Jones 1993). To obviate these complications, ionic lens materials should be allowed to neutralize for as long as possible after exposure to a peroxide-based system and only buffered saline solutions should be used with such materials.
|
|
|
|
Figure 4. Heavy film lipid deposition on the lens surface of a
silicone-hydrogel lens. Image courtesy of Brian Tompkins. |
|
Wettability
Most agree that patient perception of comfort is intimately linked to the ease with which the tear film spreads over the front surface of the lens. This surface layer helps to lubricate the interaction between the lens surface and eyelid. However, the frictional forces encountered with hydrogel lenses has been only superficially studied due to the complexities involved in measuring such low levels of friction (Nairn 1995).
All hydrogel contact lenses exhibit a gradual reduction in both comfort and wettability over time (Pritchard 1996, Boswall 1993, Marshall 1992), with lenses that are replaced frequently (disposable lenses) producing enhanced subjective performance as compared with traditional materials (Poggio 1993). The ultimate option for frequent replacement (single-use, daily disposable lenses) provides substantially improved wearing times, comfort and long-term clinical performance compared with both monthly disposable and non-frequent replacement materials (Jones 1996, Nilsson 1995, Nason 1994). The reasons behind such improvements in subjective performance and reduction in clinical complications are almost certainly due to reductions in material deposition and the maintenance of material front-surface wettability (Jones 2000, Jones 1996, Patel 1996). However, the exact reason why these effects should have such impact on clinical performance remains unclear.
In-eye wettability of hydrogels by the tear film remains extremely complex, as hydrogels are inherently hydrophobic and require a biocompatible surface to be rapidly deposited on the lens surface to make them "wettable" and tolerable (Holly 1994, Guillon 1997). This is reportedly the reason why newly-inserted lenses take some hours to become maximally comfortable, with the time taken highly patient dependent (Guillon 1997). It is possible that the tear film-derived biofilm initially deposited on hydrogel lenses reduces frictional forces between the lens and ocular surface, enhancing initial comfort. Subsequent deposition of tear-film derived material serves only to reduce wettability (Patel 1996, Jones 1997), probably due to denatured protein (Jones 1996) or increasing accumulation of non-wetting lipid (Jones 2000). This produces areas of hydrophobicity and results in the deposition and comfort problems previously described. Support for this hypothesis comes from work concerning other biomaterials. Deposition of biological fluids such as protein and lipid on articular cartilage plays a role in modifying the friction associated with natural and hydrogel surfaces (Forster 1999, Ateshian 1997, Williams 1993, Pickard 1998, Swann 1972, Little 1969, Hills 1984).
Work to date indicates that hydrogel wettability in-eye is a patient-dependent phenomenon rather than material-related, with similar levels of wettability seen in individual patients, regardless of the combination of contact lens material or care regimen (Jones 1997). One consistent factor is that wettability reduces over the period that the lens is worn (Jones 1996). Wettability of silicone hydrogel materials is similar to that of conventional lens materials and shows an identical trend to conventional hydrogels, with a reduction in wettability occurring over a period of a month (Dumbleton 2002)
If patients do exhibit reductions in wettability, then changing to another lens material will likely have a minimal impact. Such patients are best managed by switching to lenses that are replaced more frequently and, ideally, fitted with daily disposable lenses.
Conclusions
A basic knowledge of contact lens material chemistry is critical to a clinician's understanding of material behavior and significantly helps in the management of a number of clinical complications frequently seen in clinical practice. The material chemistry affects both surface and bulk characteristics, and a basic understanding of the way in which materials behave will prevent some unexpected surprises when changing lenses between FDA groups.
Novel silicone hydrogel lens materials represent a whole new class of materials and behave entirely different from conventional hydrogels. Their high levels of oxygen transmissibility ensure their future, whether continuous wear as a concept is successful or not. Complications associated with their high modulus will decrease as manufacturers develop newer materials with levels of stiffness close to that of conventional hydrogels. Future designs will benefit from changes to the manufacturing and surface coating processes, which will increase the rate of production and produce lenses at a lower cost. These changes will offer the practitioner and patient a wider choice of contact lens materials and designs in order to achieve successful continuous and daily wear. These developments will likely result in conventional hydrogel lens materials being obsolete within the net 10 years. In the meantime, clinicians should strive to remember some of the basic differences between materials such that they can manage their patients appropriately.
The author wishes to thank Dr. Kathy Dumbleton and Caroline Karlgard at the University of Waterloo for their help with the production of this article.
To receive references via fax, call (800) 239-4684 and request document 86. (Have a fax number ready.)








