GP insights
Get Involved With
GP Continuous Wear
BY LORETTA B. SZCZOTKA, OD, MS, FAAO
Using GP lenses for extended wear is not a new concept. The Berkeley Contact Lens Extended Wear Study (CLEWS), which started in 1994, determined that compared to medium Dk lenses, high-Dk lenses had a significant impact on maintaining successful extended wear. Therefore innovations in hyper Dk GP materials should only increase our success with the overnight use of GP lenses.
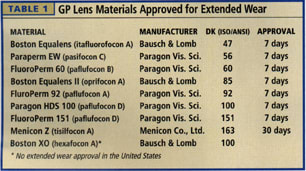
Whether you prescribe extended wear lenses for orthokeratology or for refractive error correction for seven days or 30 days, you should know your lens material options and the Dk differences between them (Table 1).
Predicting the Risks
The greatest concern with continuous wear contact lens use is microbial keratitis (MK). Since the introduction of 30-day continuous wear hydrogel and GP lenses, the clinical and research communities, the media and the FDA have closely monitored this risk -- especially considering the results of the Contact Lens Institute sponsored study in 1980, which reported that the relative risk of MK among extended wear users was 12 to 15 times that of daily wear soft users.
In recent 30-day continuous wear, hyper Dk, pre-market studies involving CIBA Vision's Focus Night & Day, Bausch & Lomb's PureVision and Menicon's Menicon Z lenses, the FDA attempted to use infiltrative keratitis as a surrogate end point for MK because of the low incidence of MK.
Because the incidence of MK is too small to reliably determine the risk in these conventional pre-approval studies, each manufacturer is now required to conduct a post-approval study to gather additional data regarding the risk of MK and subsequent loss of best corrected acuity.
If you're interested, you can get involved in a GP continuous wear post-approval study.
Get Yourself Involved
As a condition of the Menicon Z GP contact lens's approval for continuous wear of up to 30 days, Menicon is required to conduct a post-approval study to report the complications resulting from up to 5,000 patient/years of wear.
The study is designed largely as a survey with the investigators contacting the subjects at six-month intervals for up to two years. The investigators identify subjects who wear the Menicon Z lens for extended wear of 22 days or longer and they provide the subject prescription, demographics and contact information to the study monitor.
This study is currently recruiting investigators and subjects. For information or to participate in this first of its kind GP study, call Dr. Sakamoto, international director of Professional Relations for Menicon, at (808) 368-1214.
Dr. Szczotka is an associate professor at Case Western Reserve University Dept. of Ophthalmology and Director of the Contact Lens Service at University Hospitals of Cleveland.



