ACANTHAMOEBA STUDY
Comparing Laboratory and Environmental Acanthamoeba
This study determined which laboratory-produced strains of Acanthamoeba most resemble those that patients may be exposed to in the environment.
By Luiz E. Bermudez, MD, and Martin Wu, BS
Acanthamoeba keratitis is a potential threat to contact lens wearers, but fortunately the incidence is low (Radford 1998, Seal 1995, Stehr-Green 1989). Acanthamoeba are ubiquitous organisms that live in soil and many water sources, such as tap water, swimming pool water and pond and lake water (Meisler 1991, Moore 1990).
The organisms can exist as mobile trophozoites
(troph) in favorable conditions and differentiate into double-
walled cysts when the environment around them becomes less favorable (Bowers 1968, 1969). Acanthamoeba in the environment may cause diseases such as granulomatous amoebic encephalitis
(GAE) (Kilvington 1994) or keratitis (Stehr-Green 1989).
|
|
|
|
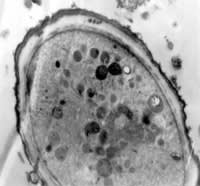
Figure 1. A. castellanii cultured in lake water for seven days; x 26
K. |
|
Acanthamoeba keratitis occurs predominantly in contact lens wearers, and the literature reflects that most affected patients contracted Acanthamoeba keratitis through contact with a contaminated water source, such as pond water, lake water, swimming pool water or tap water (Meisler 1991, Moore 1990). The cyst form is the most resistant form of the organism, but researchers do not fully understand whether the troph form or the cyst form causes Acanthamoeba keratitis (Kilvington 1990, 1994, Nagington 1976, Osato 1991, Schuster 1993). However, we can hypothesize that the cyst form found in water sources attaches to contact lenses and initiates the host infection (Perkovich 1991, Seal 1995).
Researchers can produce Acanthamoeba cysts under laboratory conditions using various methods and media. Past studies have used different methods for cyst production, but previously it was unknown which laboratory methods produce cysts that most resemble Acanthamoeba cysts in the environment. Researchers most commonly use the PYG method, the Escherichia coli / non-nutrient agar method and the starvation method (Buck 2000) to produce cysts. The PYG method, which is an axenic method, involves inoculating Acanth-
amoeba into the PYG broth, which contains positive ions such as Mg++ and Ca++, and incubating them for approximately four weeks. The organisms reproduce exponentially until they reach about 1 x 106
amoeba/mL, after which a build-up of cell byproducts, toxins and reduced nutrients causes cyst formation due to unfavorable conditions.
The E. coli / non-nutrient agar method, which is a non-axenic method, involves inoculating Acanthamoeba organisms onto non-nutrient agar plates overlaid with a thick suspension of E. coli and incubating them for approximately seven days. During this time the Acanthamoeba organisms migrate across the E. coli lawn and engulf the bacterial cells until the supply is exhausted, which causes the Acanthamoeba to begin encystation due to the lack of a food source.
|
|
|
|
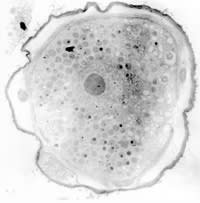
Figure
2. A. castellanii cultured in PYG for 28 days; x 18.2
K. |
The starvation method, which is also an axenic method of producing cysts, involves growing the Acanthamoeba organisms in a nutrient broth for about five days and then transferring the trophs to a medium without nutrients, such as Neff's encystment media or Page's saline, for an extended time. The lack of a nutrient source causes the organisms to encyst. We used the starvation method (14 days in PYG then 14 days in Page's saline) here as a control.
This study compares the morphology and resistance of Acanthamoeba cysts in the natural environment to laboratory cysts produced both axenically and non-axenically. We inoculated Acanthamoeba castellanii and Acanthamoeba polyphaga into PYG, E. coli / non-nutrient agar plates or environmental water sources such as tap water, swimming pool water, pond water and lake water, and maintained them at approximately 30 degrees Celsius. These organisms represent species associated with Acanthamoeba keratitis (Ahearn 1997, Burger 1994, Hones 1986, Moore 1989, 1990, Nagington 1976, Osato 1991). We compared and contrasted the resulting organisms from the laboratory methods to the organisms produced from the environmental water sources to determine which laboratory preparations would produce cysts that most resembled Acanthamoeba cysts from natural environments such as swimming pools, lakes, ponds or tap water.
|
|
|
|
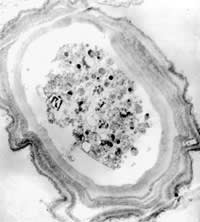
Figure 3. A castellanii cultured on E. coli/non-nutrient agar plates for seven days; x 18.2
K. |
|
Materials and Methods
Cultivation of Acanthamoeba organisms We obtained axenic cultures of A. castellanii (ATCC 30234) and A. polyphaga (ATCC 30871) from the American Type Culture Collection (Rockville, MD). We used E. coli ATCC 8739 to prepare the non-axenic cultures. The cultures of A. castellanii and A. polyphaga grew in peptone-yeast extract-glucose medium (PYG) containing 0.05% MgSO4 and 0.006% CaCl2 (Nash 1991). Phosphate buffered saline (PBS) was used to wash the organisms. We used non-nutrient agar to produce the non-axenic organisms for the experiment. Page's saline served as a control.
Stocks of A. castellanii and A. polyphaga cysts were grown in PYG for seven days, washed in PBS and grown as stated in Table 1. We added the Acanthamoeba to the appropriate media or water sources at a concentration of 1.0x105 amoeba/mL. We used a 1µ filter to filter sterilize the water sources before inoculation.
We used three laboratory preparation of cysts: Acanthamoeba maintained in PYG at 33 degrees Celsius for 28 days, Acanthamoeba maintained on non-nutrient agar overlaid with approximately 1010/mL E. coli (Ec/NNA) at 30 degrees Celsius for seven days and Acanthamoeba grown in PYG at 30 degrees Celsius for 14 days and for another 14 days in Page's saline at 20 to 25 degrees Celsius (PYG/PS).
Mechanical resistance of Acanthamoeba cysts Conditions to which we exposed samples after seven or 28 days of incubation were as follows:
- Sonication with a setting at output 1% or 10% duty cycle, pulsed for 30 seconds continually, Ultrasonic Inc., Model W-375
- Heat for 60 degrees Celsius for 20 minutes
- Ultraviolet light for 15 minutes or 30 minutes, wavelength = 365 nm, radiation of 600 mcW/cm2, Sterilaire, UVP
- Controls
Transmission electron microscopy We prepared the samples for transmission electron microscopy as follows. Acanthamoeba were detached from the flask and washed in Hank's buffered salt solution at 4 degrees Celsius. We then resuspended the pellet in ice-cold 1% glutaraldehyde in phosphate buffer for one hour and post-fixed it with OSO2 1% at room temperature for one hour. Next we centrifuged the cells and transferred them to small conical plastic capsules. Melted agar (0.7%) was added, mixed with the cells and rapidly centrifuged. We cut off the solid agar tip and dehydrated small pieces of agar that contained cells through 50% and 80% ethyl alcohol at 4 degrees Celsius.
|
|
|
|
Figure 4. A. castellanii cultured in PYG for 14 days followed by Page's saline for 14 days; x 18.2
K. |
Results
We observed that the Acanthamoeba cysts from PYG and PYG/PS most resembled the Acanthamoeba cultured from the environmental water based on their resistance to sonication, heat exposure and UV radiation. The E. coli/non-nutrient agar cysts were less resistant to sonication and UV radiation compared to the cysts from the water sources, showing approximately 20 percent and 12 percent lysis, respectively.
We observed Acanthamoeba cultures under light microscopy and transmission electron microscopy. Figures 1 through 4 show that all cysts exhibited the double-wall structure typical of mature cysts. Acanthamoeba produced in environmental water encysted by day seven and were of medium size. Samples from the Ec/NNA also encysted within seven days, but we observed that the cysts were smaller than those formed in the water sources. The Acanthamoeba cysts produced from PYG and PYG/PS were larger than those we observed from the environmental water sources. Clumping of Acanthamoeba cysts occurred in the water from both lake sources.
Discussion
Acanthamoeba cysts are characterized by thick cell walls (Bowers 1969, Neff 1964). You can see this in Figure 1 and Figure 2, which show Acanthamoeba produced in lake water and PYG media, respectively. Identifying laboratory-produced Acanthamoeba cysts that resemble those from environmental conditions is important both for studying the pathogenesis and for creating novel forms of therapy.
We know that researchers can use a variety of methods to produce Acanthamoeba cysts in the laboratory. The Acanthamoeba produced axenically by PYG and PYG/PS methods most resembled the Acanthamoeba produced from environmental water sources when compared to a non-axenic laboratory method of producing cysts. Therefore, the PYG and PYG/PS cysts most resemble what patients might encounter while swimming in a pool, lake or pond or by exposing a contact lens to tap water.
The physiological processes of encystment are still under investigation, and our results suggest that specific differences in producing Acanthamoeba influence the morphology and the resistance to stress of the resulting cysts. Przelecka and Sabota described alterations in the surface coat of A. castellanii when the trophozoite changes from the logarithmic phase to the stationary phase of growth. They observed that the amoeba lose sugar in the plasma membrane and that sugars are absent from the cysts' protoplast surface.
More recently, Coppi and Eichinger, who worked with the pathogen Enthamoeba invadens, concluded that free galactose or N-acetylglucosamine in the medium prevents encystment. In fact, galactose prevents the formation of aggregates that develop during the early phase of encystment, which suggests that functional cell surface galactose-binding molecules are present. Serum or mucin can provide galactose and N-acetylglucosamine, which indicates that there are distinct sugar-sensitive pathways that regulate differentiation of amoeba trophozoites into cysts.
Although specific environmental conditions that trigger cyst formation can be complex, our results show that cysts with similar morphology and resistance to mechanical stresses can develop through culturing Acanthamoeba in PYG medium for 28 days.
Our observations suggest that the specific factors that trigger encystment in water were present in all our samples of environmental water. We could hypothesize that some substances secreted by Acanthamoeba are necessary to initiate the process of encystment. It certainly would better explain our findings. In fact, Sykes and Band established a temporal correlation between the secretion of polyphenol oxidase and encystment of Acanthamoeba castellanii. The results of this study suggest that laboratory-prepared cysts from aqueous conditions, such as PYG and PYG/PS, most resemble resistant cysts from naturally occurring environmental conditions.
This work was supported by a grant from Alcon Research, LTD., Fort Worth, TX.
References are available upon request. To receive references via fax, call (800) 239-4684 and request document #95. (Have a fax number ready.)


|
TABLE 1 |
||
|
Acanthamoeba Cyst Growth Conditions |
||
| Media | Incubation Period | Temperature (°C) |
| PYG | 28 days | 33 |
| E. coli/non-nutrient agar plates | 7 days | 30 |
| PYG, Page's saline | 14 days each | 30, 20-25 respectively |
|
ENVIRONMENTAL SOURCES |
||
| San Francisco tap water | 7 and 28 days | 30 |
| Los Angeles tap water | 7 and 28 days | 30 |
| San Francisco pond water | 7 and 28 days | 30 |
| San Rafael pond water | 7 and 28 days | 30 |
| Lake Tahoe, CA | 7 and 28 days | 30 |
| Lake Merced, CA | 7 and 28 days | 30 |
| Swimming pool water | 7 days | 30 |