CONTINUOUS WEAR
Hyper Oxygen
Transmissible Contact
Lenses and the Epithelium
This three-study summarization looks at the effects of wear time, lens type and oxygen on bacterial binding, epithelial thickness, exfoliation and surface cell size.
By Patrick M. Ladage, BOptom, PhD, FAAO
|
|
|
|
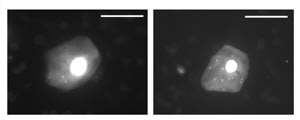
Figure 1. Irrigation chamber to collect human corneal epithelial surface cells. |
|
Ask patients with refractive errors to make a wish, and the large majority will surely answer: perfect vision for 24 hours a day and no more eyeglasses! This desire has led to a proliferation of individuals choosing corneal laser surgery. The daily bombardment of radio ads is certainly hard to ignore and confirms the place of laser vision correction in today's eyecare practice. While corneal laser surgery patients are generally very satisfied with the procedure, some unlucky ones wonder why they ended up with devastating consequences that will remain for the rest of their lives. Regardless of one's position for or against, it is important to know that laser surgery is not the only option for permanent refractive correction.
With the recent introduction of new hyper oxygen transmissible contact lens materials, 30-nights extended wear (EW) is now gaining strength as a form of (quasi-) permanent vision correction. Its distinct advantages: no cutting and slicing in healthy tissue and full reversibility (remove the lens), yet the benefit of 24 hours a day, fully corrected vision for up to a month. Certainly, in expanding EW to 30 nights, we should not disregard the lesson from the past: an increased risk of corneal infection associated with EW. Nonetheless, it is anticipated that the incidence of contact lens-induced corneal infections will decline with the widespread use of the new hyper oxygen transmissible silicone hydrogel and gas permeable (GP) contact lenses.
Based on encouraging clinical data, the FDA has therefore recently reconsidered its stance on 30-night extended wear and is now again allowing this form of contact lens wearing modality. This significant decision ensures an alternative and, more importantly, a competitive option to laser refractive surgery for those patients seeking continuous vision correction. This article reviews the results and clinical implications of three FDA-sponsored studies on 30-night extended wear, conducted at UT Southwestern Medical Center, Dallas (Ladage 2001, Cavanagh 2002, Ren 2002).
|
|
|
|
Figure 2. Low and high Pseudomonas aeruginosa binding to human corneal |
Bacterial Binding
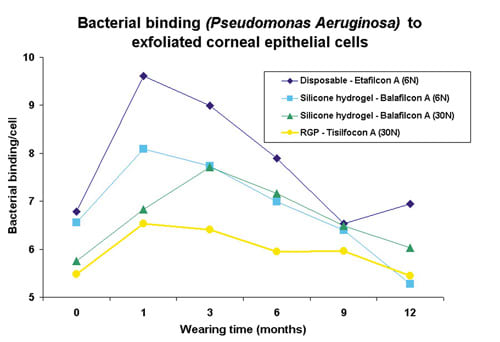
In a prospective study, the effect of wearing time (daily wear and six nights vs. 30 nights EW), lens oxygen transmissibility (high vs. hyper) and contact lens type (soft vs. GP) on contact lens-induced bacterial binding to corneal epithelial surface cells was examined for the study duration of one year. Four test lenses were used in a study group totaling several hundred patients: a high oxygen transmissible disposable contact lens (Acuvue, Vistakon), two hyper oxygen transmissible silicone hydrogel lenses (PureVision, Bausch & Lomb and Focus Night & Day, CIBA Vision) and one hyper oxygen transmissible gas permeable lens (Menicon Z, Menicon). In order to measure bacterial binding, it is necessary to harvest corneal epithelial cells. Figure 1 shows an irrigation chamber used in the clinic to rinse the cornea and collect surface epithelial cells from one eye of each patient. These collected cells were then mixed in the laboratory with Pseudomonas aeruginosa bacteria, the most common and devastating infection agent associated with lens-related microbial keratitis. Following several staining and washing procedures, the epithelial cells were visualized under a microscope, and subsequently a masked observer determined the number of adherent bacteria per cell (Figure 2) for each individual prior to and following contact lens wear.
During daily wear, the hyper O2 transmissible GP lens was the only contact lens not to cause a significant increase in bacterial binding compared to pre-lens baseline control. Of all the studied test lenses, the high oxygen transmissible disposable contact lens showed the largest increase in bacterial binding during daily wear, while the results of the hyper O2 transmissible silicone hydrogel lenses fell in between the GP and disposable lenses.
|
|
|
|
Figure 3. Pseudomonas aeruginosa binding during EW with different test contact lenses. |
|
Throughout 12 months of EW, bacterial binding with GP wear was essentially flat. Figure 3 shows the result of bacterial binding from one study (Ren 2002). By contrast, bacterial binding in the disposable and silicone hydrogel groups was elevated in the first months of EW, but surprisingly recovered at the conclusion of the study. Similar to DW, silicone hydrogel lens wear caused less bacterial binding overall during EW than the disposable hydrogel control lens. Furthermore, among the silicone hydrogel lens wearers, we saw no significant difference in bacterial binding between sleeping in the lens for six or 30 nights (Figure 3).
What does this mean? Even though bacterial binding does not necessarily equal corneal infection, it seems very indicative that the GP lens showed no significant increase in binding at all. We know that GP lens wear is currently the safest contact lens wearing modality available. The most recent epidemiological study confirmed this once again as GP lens wearers are exposed to the lowest annualized corneal infection risk rate, 1.1 in 10,000 wearers or 0.011 percent (DW) (Cheng 1999). Soft lens wearers, on the other hand, have a slightly higher annualized risk, respectively 0.035 percent (DW) and 0.2 percent (EW). The annualized rate of GP EW and silicone hydrogel lens wear (DW and EW) is yet to be determined by large-scale epidemiological studies. However, the first indications are encouraging. Based on a series of reported infections and calculated lens sales data, Holden et al estimated the current annualized incidence of infection with extended silicone hydrogel lens wear to be 0.005 percent (Holden 2003).
Another striking finding of these studies was that bacterial binding during soft lens wear is most noticeable during the first three to four months of EW, with adaptation following afterward. Therefore, monitoring new EW patients during the first months of EW certainly warrants extra attention.
Epithelial Thickness
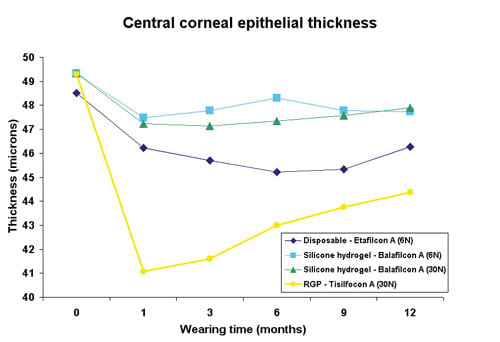
The corneal epithelium forms, together with the conjunctival epithelium, the outer layer of the ocular surface. The integrity of this layer is essential in protecting the deeper structures of the eye against infection and mechanical damage. Holden et al were the first to show in human patients that long-term EW contact lens wear significantly thins the corneal epithelium by 5.6 percent (Holden 1985). This classic paper was published before the use of hyper transmissible GP and silicone hydrogel lenses, thus the question remained: "Do these new lenses also cause a significant thinning?" We have measured with in vivo confocal microscopy the thickness of the central corneal epithelium before enrollment in the study (baseline, point 0) and during DW/EW.
|
|
|
|
Figure 4. Central corneal epithelial thickness during
EW. |
Daily wear did not cause a significant thinning of the corneal epithelium during disposable and silicone hydrogel lens wear (Ladage 2001b). By contrast, DW GP lens wear thinned the central epithelial thickness by almost 10 percent. Figure 4 shows the recent clinical results of central epithelial thickness over time, before and during EW (Ren 2002). The GP lens group had a maximum thinning of 11 percent after one month of EW. The soft lenses also caused thinning during EW with maximum decreases of 6.8 percent (disposable 6N), 3.8 percent (silicone 6N) and 4.4 percent (silicone 30N). The hyper O2 soft lenses caused significantly less thinning than the disposable control lens. The difference between silicone six nights and 30 nights was not statistically significant. Note that there was not much difference in thinning in the GP group between DW and EW (10 percent vs. 11 percent) while the soft lenses exhibited significant thinning entirely during EW. Two likely mechanisms may explain this: mechanical and/or metabolic thinning. The thinning associated with hyper O2 transmissible GP lenses is largely of mechanical nature, similar to the reshaping effect of the cornea seen with orthokeratology lens wear. Central pressure induced by GP contact lenses can rather quickly redistribute the epithelial cell mass from the center toward the periphery (Swarbrick 1998). The thinning induced by soft lens wear, however, may be metabolic. Animal research has shown that the rate of cell division is decreased during EW and that the lowest oxygen transmissible lenses cause the largest inhibition, up to 81 percent with Dk/t lenses of 15 (Ladage 2001, Ladage 2003). A decrease in cell division will lead to a slower and more gradual thinning of the corneal epithelium until a new homeostatic equilibrium is achieved. Interestingly, a partial recovery in corneal epithelial thickness was visible over time, suggesting adaptation of the corneal epithelium to the new conditions (Figure 4).
|
|
|
|
Figure 5. Corneal surface cell exfoliation rate during
EW. |
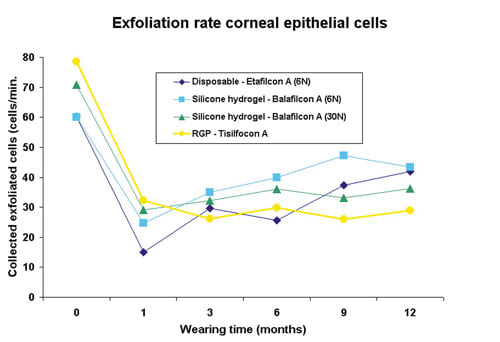
Epithelial Surface Cell Exfoliation
Every day, the corneal epithelium is continuously losing surface cells into the tear film through a process called exfoliation. Apoptosis, a form of cell suicide, is believed to regulate this process as mostly single cells are being deleted from the ocular surface in an orderly manner (Ren 1996). Exfoliation of surface cells into the tear film is part of the ocular defense system, which makes it more difficult, for example, for bacteria adherent to surface epithelial cells to stay for prolonged periods of time. The surface epithelial exfoliation rate (cells/minute) can be estimated prior to lens wear and after DW/EW using the same irrigation chamber as in the bacterial binding experiments. Figure 5 shows that all lenses decreased the exfoliation rate of the corneal surface, regardless of lens modality, type or lens oxygen transmissibility. This strongly suggests the physical presence of the lens on the ocular surface prevents or delays the exfoliation of cells into the tear film. Comparable observations have been made in animal research as rabbits wearing EW lenses show decreased apoptotic and dying cells on the corneal surface (Yamamoto 2001, 2002). One possible explanation is that the eyelids induce forces as they slide over the cornea during each blink and initiate the process of surface cell exfoliation. A lens positioned between the cornea and the eyelids may serve as a buffer, partially absorbing the shear forces of the eyelids.
|
|
|
|
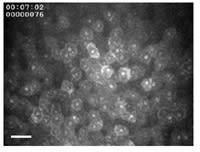
Figure 6. Frontal view corneal epithelial surface as seen by in vivo confocal
microscopy. Bar=50 µm. |
|
Epithelial Cell Size
As epithelial cells move from the basal cell layer to the surface, their cell bodies progressively flatten into thin superficial epithelial cells with large surface sizes. Long-term extended wear is known to further increase the size of surface epithelial cells. It has been hypothesized that a slowing of the corneal epithelial renewal rate causes this increase in surface size: cells remain longer on the surface (they do not come off as easily) and therefore continue to enlarge as they get older (Lemp 1986). Figure 6 illustrates the front view of the corneal epithelial surface as seen by in vivo confocal microscopy. Corneal surface images from all participating study patients were digitized at different timepoints, and a masked observer then measured with specialized computer software the average epithelial surface cell size.
Disposable and silicone hydrogel DW and EW caused a similar significant cell size increase (Ladage 2001, Cavanagh 2002, Ren 2002). The largest and most remarkable increase was seen with the GP lens, in DW and EW. Parallel to epithelial thinning, this increase in cell size occurred rapidly and was thereafter fairly constant. It appears from this observation that at least one additional mechanism, mechanical pressure, in addition to the hypothesized aging can cause an enlargement of surface cell size.
Summary
The era of chronic lens-induced hypoxia, whether in daily or extended wear, is coming to an end thanks to hyper oxygen transmissible soft and GP lens materials. Clinical complications associated with hypoxia such as neovascularization, limbal redness, corneal edema and epithelial microcysts are on the decline or have even disappeared (reviewed in Silicone Hydrogels The Rebirth of Continuous Wear Contact Lenses, editor D. Sweeney). At the cellular level, there is less lens-induced bacterial binding to surface epithelial cells, less decreases in epithelial cell proliferation and less epithelial thinning. Taken together, all these findings suggest that corneas are healthier during hyper O2 transmissible lens wear as compared to corneas exposed to lower O2 transmissible lenses. Is this the end of the story? No. Eliminating hypoxia solves a lot of problems and is a great advance for lens wearers and practitioners, but we still have to deal with other areas that may contribute to the development of corneal infection. This includes the stagnation of the tear film (particularly soft lenses), reduced surface epithelial cell exfoliation and epithelial trauma. Nevertheless, maintenance of a healthier ocular surface during hyper O2 transmissible contact lens wear should reduce the danger of infection. Overall, hyper O2 transmissible GP lenses, followed by silicone hydrogel lenses, appear to be the best first choice in terms of safety for new contact lens patients and those needing lens replacements.
The author has no financial interest in the products mentioned.
To receive references via fax, call (800) 239-4684 and request document #94. (Have a fax number ready.)