KERATOCONUS STUDY
Searching for Comfort in a GP Keratoconus Lens
This keratoconus GP lens design may provide comfortable lens wear to your keratoconus patients.
By Pat Segu, OD, Julie Jackson, RN, OD, William Miller, OD, PhD, Norman Leach, OD, MS, and Jan
Bergmanson, OD, PhD
Keratoconus is a noninflammatory, usually bilateral disease that results in corneal thinning and ectasia. Keratoconus affects approximately one in 2,000 people, which makes it a disease that we are likely to encounter. Keratoconus is the most common noniatrogenic corneal pathology to require keratoplasty as a result of scarring, contact lens intolerance or poor visual acuity. Because of the visual distortion resulting from the corneal ectasia, adequate visual correction in moderate to severe keratoconus patients often requires gas permeable (GP) contact lenses.
Because the central cornea progressively becomes steeper and thinner, contact lens fitting for keratoconus patients is often challenging. Not only is the fitting process for keratoconic corneas cumbersome and time consuming, but sustained comfort and perceived visual performance are additional compounding issues for this patient population.
Technology Eases a Difficult Fit
Fortunately because of advances in GP lens design, keratoconus patients may be able to delay surgical intervention. The purpose of this study was to evaluate the clinical viability and the subjective performance of the FDA-approved Metro Optics ComfortKone keratoconic lens design fit on a population of experienced GP-wearing keratoconus patients.
The ComfortKone contact lens is a triaspheric GP lens design that features a 4.0mm wide spherical optical zone to fit the central corneal region affected by keratoconus. Other parameters for this lens include diameters of 7.5mm to 9.5mm in 0.1mm steps, base curves from 4.5mm (75.00 diopters) to 8.0mm (42.00 diopters) and any sphere power. Custom powers are also available. The ComfortKone lens provides maximum alignment centrally while the aspheric peripheral curves (denoted as A values) are designed to fit the abnormal corneal slope created by keratoconus. The A values range from A3 to A20 (in steps of 1), in which a smaller A value indicates a smaller change between the base curve and peripheral curves to contour the abnormal corneal slope between the cone and mid-peripheral cornea.

The Texas Eye Research and Technology Center (TERTC) at the University of Houston College of Optometry conducted a six-month prospective study designed to evaluate the visual status and overall comfort of the ComfortKone lens in an established population of keratoconic subjects. In this study, which received approval from the University of Houston Committee for the Protection of Human Subjects, we enrolled a total of 10 consenting keratoconic subjects who were currently wearing GP lenses (but not ComfortKone).
We refit each subject enrolled in the study into the ComfortKone lens using a fluorosilicone acrylate (Dk=30) lens material. To promote adequate tear exchange and reduce physical pressure over the ectactic cornea, we incorporated a previously published (Jackson et al April 2003) peripheral curve radius bracketing technique to fit the ComfortKone lens. This technique involves selecting an initial diagnostic lens based on the average of the flat and steep keratometric readings. We then altered the base curve until we achieved slight apical vaulting (first definitive apical clearance lens as defined by the Collaborative Longitudinal Evaluation of Keratoconus [CLEK] study group). We selected the A value of the ComfortKone lens by consistently beginning with an A value of A7 or A10, then bracketing the A value by incremental steps of 5 until we achieved adequate tear exchange and an acceptable mid-peripheral pattern.
|
|
|
|
Figure 1. Evaluation of the ComfortKone lens using
biomicroscopy. |
|
We required all subjects to fill out a questionnaire that addressed overall lens comfort, lens sensation, lens placement, dryness, itching and burning. We used a Likert scale from 0 to 100 (in which 100 indicated complete comfort) to rate each category. Additionally, we used biomicroscopy (Figure 1), corneal topography (OrbScan), pachymetry (OrbScan), Snellen visual acuity and contrast sensitivity (Pelli-Robson) to monitor the study population.
Results
Currently seven subjects have completed the study and we have analyzed their six-month patient questionnaire data and documented the following keratoconus characteristics for each subject: steepest meridian, corneal scarring, Fleischer's Ring, Munson sign and prominent corneal nerves (Table 1). Based on the classification used in the CLEK study, all seven subjects had at least one eye in the moderate (45.00 diopters to 52.00 diopters) or severe (>52.00 diopters) level of keratoconus. We assessed corneal thickness values with the help of an Orbscan Topographer (Table 1). The average apical (cone) corneal thickness for all seven subjects was 0.417mm. Nine of the 14 eyes exhibited Fleischer's Ring, only four eyes demonstrated Munson's Sign and two eyes had corneal scarring within 6mm of the central cornea. Prominent corneal nerves were evident in both eyes for all seven subjects.
The corrected Snellen visual acuity with the ComfortKone lens ranged from 20/20 to 20/30. The mean contrast sensitivity for the seven subjects was 0.27 log MAR with the ComfortKone lens.
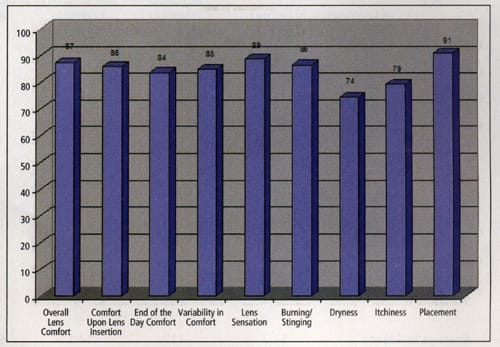
The results of the six-month patient questionnaire for the seven completed subjects reveal that the average score for overall lens comfort for all eyes was 87 (± 17 SD), with a range from 55 to 100. Comfort upon lens insertion scored on average 86 (± 23 SD) for all eyes, ranging from 27 to 100. The average score for end-of-day comfort for all eyes was 84 (± 22 SD) with a range of 35 to 100. Variability in comfort during the day scored 85 (± 26 SD) for all seven subjects, ranging from 25 to 100. The average score for lens placement was 91 (± 15 SD) with a range of 75 to 100. The CLEK study reported that 73 percent of keratoconic patients corrected with GP lenses considered their contact lenses comfortable. The initial results of our study optimistically appear to indicate satisfactory comfort with the Metro ComfortKone lens.
|
|
|
|
Figure 2. Average scores for completed subjects at the six-month
visit. |
The patient questionnaire also asked each subject to rate lens sensation, burning/stinging, dryness and itchiness (Figure 2). Our patient population reported dry eye complaints that the literature has commonly identified with keratoconus and may be related to other conditions such as atopy.
Conclusion
Preliminary results for corrected visual acuity and overall subjective comfort with the Metro Optics ComfortKone GP contact lens are encouraging for this keratoconus patient population. Fortunately, advances in GP contact lens designs that use lathe technology allow better edge profiles and more complex design solutions that result in overall improvement with lens performance. Such advancements are instrumental for eyecare practitioners faced with the fitting challenges presented by keratoconus patients. Our data suggests the Metro Optics' ComfortKone lens allows practitioners to achieve an acceptable physiological fit while at the same time provides the keratoconic patient satisfactory overall comfort and corrected visual acuity.
References are available upon request. To receive references via fax, call (800) 239-4684 and request document #98. (Have a fax number ready.)
Dr. Segu is a clinical associate professor at the University of Houston College of Optometry and is the director of optometry services at the Good Neighbor Healthcare Center.
Dr. Jackson is the director of Student Affairs and is a clinical assistant professor at the University of Houston College of Optometry.
Dr. Miller is an assistant professor at the University of Houston College of Optometry.
Dr. Leach is a clinical professor and the director of the Cornea and Contact Lens Service at the University of Houston College of Optometry.
Dr. Bergmanson is a professor of optometry at the University of Houston College of Optometry where he is also the founding director of the Texas Eye Research and Technology Center (TERTC).