continuous wear colloquy
The Future of
GP Continuous Wear
BY LORETTA B. SZCZOTKA, OD, MS, FAAO
Silicone is the magic ingredient in continuous wear soft lenses that dramatically increases their oxygen permeability (Dk) over low-Dk hydrogels. Is this the case for extended or continuous wear GP lenses? The answer is no. In GP material chemistry, significant tradeoffs, including increased deposits, poor surface wetting, increased lens flexure, material instability and poor manufacturing capability, result when manufacturers use increased silicone to increase lens permeability.
Finding Other Alternatives
Over the past 25 years, traditional GP silicone methacrylate backbones and silicone contents have seen a downward trend as chemists have found other methods of improving oxygen permeability.
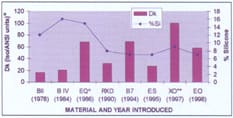
For example, in Polymer Technology's line of GP products, you can see a clear shift toward lower silicone concentration while Dk increased (Figure 1). In earlier generation lenses, oxygen permeability resulted from numerous silicone groups hung off the polymer chain, creating free volume space. In 1995, Polymer Technology introduced their AERCOR chemistry approach to increased oxygen permeability using non-silicone containing backbones that are innately oxygen permeable.
|
|
|
|
Figure 1. Polymer Technology material changes through the years. |
|
Purifying Silicone
Paragon began using "purified silicone" in 1997 in its Hyperpurified Delivery System (HDS) series of materials. Based on NASA research, scientists first hyperpurify silicone to diminish its detrimental effects of poor wetting and dimensional instability. This results in fewer silicone-containing constituents and maximum methylmethacrylate in the polymer backbone. The final lens has mid to high oxygen permeability with hardness and machinability characteristics similar to materials in the next lower class of Dk values. This series contains HDS, Paragon Thin and HDS 100^.
Building Stronger Materials
Menicon has improved both oxygen permeability and material strength by adding novel siloxanylstyrene monomers into the polymer backbone of its Menicon Z^ lens. It's the first GP material classified in the "hyper-oxygen transmissibility" category with a Dk of 175.1*, and it's the only GP lens that is FDA-approved for 30 days of continuous wear.
Unlike its previous lenses (SFP and EX), which relied on silicone-containing methacrylate compounds for enhanced oxygen permeability, Menicon Z features Tris (trimethylsiloxy) silyl styene (SiSt) as the key monomer to fluoromethacrylate. This chemical structure results in excellent mechanical properties, allowing the lens to be significantly thinner than a typical GP lens. For example, the impact resistance of Menicon Z at a center thickness of 0.13mm is similar to a Dk of 60 at a thickness of 0.15mm.
* ISO/ANSI units, using ANSI Z80.20/ISO 9913-1 polarographic method corrected for boundary layer and edge effects (Benjamin 2002).
** Manufacturer reported Dk.
^ Material is approved for extended or continuous wear.
Dr. Szczotka is an associate professor at Case Western Reserve University Dept. of Ophthalmology and Director of the Contact Lens Service at University Hospitals of Cleveland.