MICROBIAL KERATITIS AND SILICONE CLS
Microbial Keratitis and Silicone Hydrogel Lenses
A look at the contributing factors, precautions and patient management issues with this complication.
By Katie Edwards, BOptom, Garry Brian, MBChB, FRACS FRANZCO, Serina Stretton, BSc, PhD, Fiona Stapleton, BSc, MCOptom, PhD, FAAO, Mark D.P. Willcox, BSc, PhD, Padmaja R. Sankaridurg, BOptom, PhD, Deborah F. Sweeney, BOptom, PhD, FAAO, and Brien A. Holden, BSc (Appl Sci), PhD, DSc,
FAAO
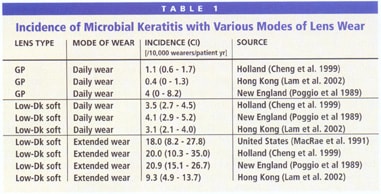
Microbial keratitis (MK), the most serious contact lens-related adverse event, can occur with any type of contact lens, but historically is more prevalent with overnight wear of soft lenses made from materials of low oxygen permeability (Dk) (Table 1). High-Dk silicone hydrogel lenses give patients a convenient, comfortable and nearly hypoxia-free alternative to other forms of vision correction. Still, we must remain prepared to manage any adverse events, including MK, that may occur with these lenses.
We aim to summarize what is currently known about cases of MK with silicone hydrogels and to provide broad guidelines for diagnosis and treatment of contact lens-associated MK, with particular emphasis on differentiation of MK from contact lens-induced peripheral ulcer (CLPU).
Learning More about MK
As a condition of the US Food and Drug Administration (FDA) approval for 30-day wear of silicone hydrogel lenses, CIBA Vision and Bausch & Lomb are conducting studies to gather data regarding the risk of MK. These studies will provide the earliest estimate of the incidence of MK with 30-day wear of silicone hydrogels as well as information on risk factors and wearer behavior. However, they won't assess the risk of MK with silicone hydrogel lenses worn on shorter wear schedules or for daily wear.
The Cornea and Contact Lens Research Unit (CCLRU), School of Optometry and Vision Science, University of New South Wales (UNSW), Australia is currently collating anecdotal cases of MK with high-Dk silicone hydrogel lenses. As of November 2003, we've collated 53 cases reported with silicone hydrogel lenses worldwide.
Etiology
The risk of MK associated with low-Dk soft extended wear has been sufficient for practitioners to discourage patients from wearing this form of correction for more than two decades. The assumption is that lens-induced hypoxia creates an environment that results in an epithelium that is more susceptible to the adherence of bacteria and consequent infection. Some evidence for this comes from animal models with low-Dk lenses showing that overnight wear causes a loss in adhesion between epithelial cells that results in poor barrier function (Madigan and Holden, 1992), and from Cavanagh and colleagues' (2002) studies of the adherence of Pseudomonas aeruginosa to exfoliated cells from the corneal surface of low- and high-Dk soft lens wearers. Several studies have shown that continuous wear with high-Dk soft lenses results in a less pronounced effect on the epithelial surface compared to extended wear with low-Dk soft lenses (Cavanagh et al 2002, Ren et al 2002, Keay et al 2000, Dumbleton et al 2001).
To date, clinicians have hypothesized that a lens that eliminates hypoxia would maintain a healthy, infection-resistant epithelium and significantly reduce the potential for MK. Therefore the improved oxygen transmissibility of silicone hydrogels theoretically should result in a lower risk of MK compared to soft lenses made from lower-Dk materials.
Signs and Symptoms
MK is an infection of the cornea characterized by excavation of the corneal epithelium, Bowman's layer and stroma, with inflammation and necrosis of tissue. It can be difficult to distinguish between MK and CLPU, which is an inflammatory reaction of the cornea that, in its active stage, is characterized by focal excavation of the epithelium, underlying inflammation and necrosis of the anterior stroma and an intact Bowman's layer. It's non-infectious and is associated with transient colonization of contact lenses by Gram-positive bacteria (Jalbert et al 2000, Willcox et al 2000). We can often differentiate the two conditions by clinical presentation (Table 2). MK is characterized by a progressive worsening of signs and symptoms, whereas CLPU is self-limiting, and often patients who have CLPU will recover without medical intervention. However, it's essential that you always closely monitor these patients and adopt a conservative approach to management.
We may be first alerted to MK by a patient's symptoms. Rapid onset of moderate to severe pain, marked redness, discharge, tearing, photophobia, decreased vision and swelling of the lids, all of which worsen without treatment, are typical. In contrast, CLPU usually presents with tearing, no lid edema, and only moderate and localized redness. Patients who develop MK while wearing silicone hydrogel lenses experience these symptoms: pain, redness, photophobia, reduced vision and lacrimation.
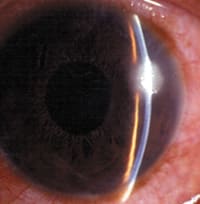
On examination, MK often has a relatively large, irregular, central, paracentral or peripheral lesion (Figure 1a), which may be accompanied by diffuse infiltration and small satellite lesions. However, the clinical picture will depend on the stage at which the patient presents, and early lesions may be small. MK may involve just the anterior stroma or the entire corneal thickness, and an anterior chamber reaction is usually present.
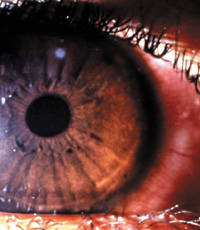
In contrast, CLPU is almost always characterized by a small, circular, focal infiltrate in the periphery to mid periphery (Figure 1b), which may be surrounded by slight diffuse infiltration. CLPU usually extends only to the anterior stroma, and anterior chamber reaction occurs only in severe cases. Treat any case that has a central infiltrate and overlying loss of epithelium, or that has irregular, focal infiltrates with raised edges or satellite lesions, as MK until proven otherwise.
The signs of MK seen with silicone hydrogel lenses reported to date are similar to those caused by low-Dk soft contact lenses. In the 13 cases of MK with silicone hydrogels of which we are aware, and where the size of the lesion was specified, 11 were 2mm or smaller in diameter. This may be because patients in all lens wear modalities now tend to present earlier to their practitioners upon noticing symptoms.
|
|
|
| Figure 1a. MK (Pseudomonas spp.) associated with soft contact lens wear. | Figure 1b. Typical appearance of an active contact lens-induced peripheral ulcer. |
| Reprinted with permission, Sweeney, et al. Clinical Characterisation of Corneal Infiltrative Events Observed with Soft Contact Lens Wear. Cornea. 2003; 22(5):435-442. | |
Risk Factors
Traditionally, the risk factors associated with MK and low-Dk soft lens wear include longer periods of uninterrupted overnight wear, use of contaminated lens care solutions and products, overnight use of daily wear lenses, male gender, lower socio-economic groups, smoking and diabetes (Stapleton et al 2003, Ormerod et al 1987). In addition, it's possible that low levels of ocular pathogens in water supplies and in hot tubs may place lens wearers at risk (Willcox et al 1997).
Known risk factors also could be associated with some of the patients who developed MK while wearing silicone hydrogel lenses. Non-compliance or inappropriate wear schedule was reported in 10 of 24 wearers for whom wear schedule was known. Hygiene was poor in three of 22 wearers where hygiene procedures were reported. Information on whether wearers swam in their lenses before the event was available for 13 cases. Of these, five reported no swimming before the event and eight reported swimming without goggles. Three wearers disinfected their lenses after swimming and five did not.
In 25 cases where information on age was available, 18 patients were under 30 years of age, and of the 42 where gender information was available, 21 were male and 21 were female. Recently McNally and colleagues (2003) have found that people with a history of inflammatory events or those aged between 18 and 29 years were at greater risk of developing corneal infiltrates while wearing silicone hydrogels during continuous wear.
Managing Patients
Educate patients to take early action in the case of an adverse event. Inform them to remove their lenses immediately if they experience irritation, discomfort, pain, redness, discharge, tearing, photophobia, decreased vision or swelling of the lids. Above all, patients shouldn't sleep in their lenses if they don't feel well, particularly if they have an upper respiratory tract infection, and they should cease lens wear if the problem is severe. Remind patients to contact you as early as possible in the case of adverse events or any symptoms of concern. Studies have found that a delay in the diagnosis and treatment of MK may result in perforations or the need for penetrating keratoplasty.
Warning patients that risk factors exist and encouraging them to avoid these risk factors is the first line of defense against MK and other infiltrative conditions. Have patients sign a record of informed consent to emphasize the risk of potential complications. Also remind patients of the importance of compliance to wear schedule and strongly discourage them from trying to lengthen the life of their lenses beyond 30 days. Remind them to regularly change lens care solutions and storage cases, even if it's only every 30 days. Warn smokers of the increased risk they have of infection and advise patients to wear tight-fitting goggles when swimming with lenses.
Continuous wear patients may fall into the habit of relying solely on contact lenses. Advise them to keep an up-to-date pair of spectacles available. If patients need to remove their lenses for any reason, stress that they thoroughly clean and disinfect the lenses before reapplying them.
Bacteria are the most common microorganisms associated with infections during contact lens wear for correction of low-refractive errors, followed by protozoa (Acanthamoeba), then fungi (Table 3). The most commonly associated species are the Gram-negative bacterium, P. aeruginosa, and the Gram-positive coagulase negative Staphylococci, Staphylococcus aureus and Streptococcus pneumoniae (Galentine et al 1984, Schein et al 1989). The spectrum of microorganisms isolated from the known cases of keratitis with silicone hydrogel lenses are similar to those seen with low-Dk soft contact lenses. Clinicians have isolated Gram-negative bacteria from 12 of the 15 cases of culture proven MK with continuous wear of silicone hydrogels, Gram-positive bacteria from three cases and fungi from one case. The most predominant bacteria isolated were P. aeruginosa (n = 8) followed by alpha-hemolytic streptococci (n = 2).
Treating the Patient
In most cases, practitioners treat contact lens associated MK empirically and will choose an aggressive antibiotic regimen based on the nature of ulceration (Figure 2). They then modify strategies depending on the patient's response and/or after identifying the species of the isolate and its antibiotic susceptibility. Be vigilant because early detection of a failure to respond to initial therapy is vital for a good visual outcome. If a practitioner doesn't have access to therapeutic drugs or the legal authority to prescribe them, then he should refer the patient to a practitioner who does, or to an ophthalmic emergency room. Provide the patient's contact lens, lens case and lens care solutions (if available) and clear and suitable documentation stating the patient's contact lens modality to facilitate appropriate treatment.
Try one of these strategies to treat MK:
- Topical fluoroquinolone monotherapy for small, non-severe ulcers (<2mm with infiltrates in the anterior to mid stroma)
- Topical fluoroquinolone in combination with a fortified antibiotic for larger (>2mm) and more severe ulcers. Cephalozin is the preferred agent against Gram-positive bacteria if there is a possibility of S. pneumoniae or if Gram-positive isolates are resistant to fluoroquinolone
- Topical fortified aminoglycoside in combination with topical fortified cephazolin for ulcers that have not responded to initial treatment
We may sometimes use adjunct therapies such as oral doxycycline if the ulcer is large and there is corneal thinning. Use cycloplegics in all patients to alleviate symptoms and to prevent formation of posterior synechiae. Because topical corticosteroids may hinder eradication of pathogens, we rarely use them for the acute management of MK. Some practitioners choose to prescribe steroids to reduce inflammation and to improve comfort for their patients once the epithelial defect has resolved.
Take cultures in severe cases of ulceration where lesions are larger than 2mm in diameter, are more than one-third of the corneal thickness or are in the central optical zone. Also culture patients who are immunocompromised, monocular, elderly or have had previous ocular surface disease or an unusual history or clinical findings, as it's essential that these patients receive appropriate targeted treatment.
Take cultures from the contact lens, case and solutions because any bacterial species found on these can indicate the potential presence of some causative microorganisms such as Acanthamoeba. If the patient is wearing the lens, have him remove it straight off the cornea, preventing contamination from lids and lashes, and place it into a new case with a small amount of saline or artificial tears (preferably from a sterile bottle). Send this to the lab with the old lens case and used solutions (if available).
Always alert the lab that incoming samples are from a lens-related infection and before culturing to ensure that they process samples immediately upon arrival. If you suspect Acanthamoeba, then consult the lab so that it processes the sample appropriately. The lab should test all samples for the presence of bacteria or fungi in the event that initial diagnosis is incorrect.
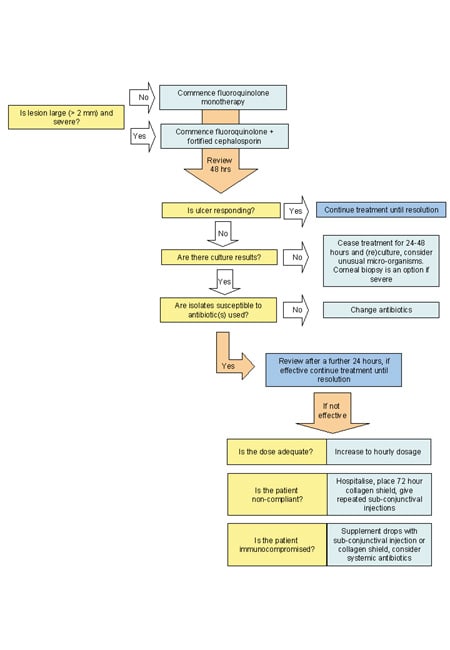
Figure 2. Management
algorithm for contact lens-associated
bacterial keratitis.
Adapted from Hodge, WG, and Hwang, DG. Antibiotic Use in Corneal and External Eye Infections. Focal Points, American Academy of Ophthalmology 1997; 15(10).
Fluoroquinolone Monotherapy
The most commonly used fluoroquinolones for treatment of MK (ciprofloxacin, ofloxacin, levofloxacin) have shown great success, despite their limited activity against Streptococcus spp. However, inappropriate fluoroquinolone use increasingly contributes to emerging resistance and ultimately clinical failure. Increasing numbers of isolates of S. aureus and coagulase-negative Staphylococci are resistant to ciprofloxacin and ofloxacin (Goldstein et al 1999), and some have reported resistance by P. aeruginosa (Chaudhry et al 1999). Levofloxacin has broader spectrum activity against Gram-positive bacteria but in-vitro tests indicate that it's not more effective against Gram-negative bacteria, particularly P. aeruginosa, compared to ofloxacin and ciprofloxacin (Kowalski et al 2001). The latest (fourth) generation of fluoroquinolones (moxifloxacin, gatifloxacin) are becoming available for treating MK. In-vitro testing with these fluoroquinolones against bacterial endophthalmitis isolates indicates that they have greater potency against a range of Gram-positive bacteria and have activity against Gram-positive isolates already resistant to ciprofloxacin and ofloxacin. The activities of moxifloxacin and gatifloxacin against the Gram-negative endophthalmitis isolates were similar to ciprofloxacin (Mather et al 2002).
Reserve fluoroquinolone monotherapy for less severe cases of ulceration. Evidence from the ofloxacin study group suggests that lesions less than 2mm2 are unlikely to be culture positive, and therefore culturing in these cases does not yield any information that will aid treatment (Morlet and Daniell 2003).
Commence fluoroquinolone monotherapy immediately. If the condition rapidly worsens, then modify treatment based on the clinical response and, if available, culture results (Figure 2). If the lesion responds within the first two days, then continue therapy until the eye becomes quiet.
Adverse reactions to fluoroquinolones are rare but may include discomfort on instillation, stinging, tearing, hyperemia, skin rash or dizziness. Ciprofloxacin has been associated with white crystalline precipitates in the corneas of some patients.
Resuming Lens Wear
Patients can resume lens wear once all signs of active inflammation disappear and the condition has completely resolved. Attempt to address any risk factors for MK that patients may have before they resume lens wear. Recommend lenses and modalities that are less likely to cause infection for patients that are keen to resume lens wear but have one or more risk factors for infection.
Summary
Make sure that patients don't become complacent with lens wear, particularly continuous wear. You and your patients need to know the risks and early signs of MK. Early detection, referral and treatment are essential to reduce the morbidity of MK. While the risks of MK may seem relatively small, the numbers of lens wearers worldwide is increasing. Therefore, the potentially severe ocular morbidity associated with such infections is an important health concern. We owe it to our patients to understand what causes these events, to actively educate them on how to avoid these events and to be able to promptly diagnose and treat any cases.
To receive references via fax, call (800) 239-4684 and request document #101.
|
TABLE 2 Differential diagnosis of MK and contact lens-induced peripheral ulcers* |
||
| Microbial Keratitis (MK) | Contact lens-induced peripheral ulcer (CLPU) | |
| DEFINITION | Microbial infection characterized by excavation of the corneal epithelium, Bowman's layer and stroma with infiltration and necrosis of tissue | Inflammation of the cornea characterized in its active stage by focal excavation of the epithelium, infiltration and necrosis of the anterior stroma. Bowman's layer is intact. |
| OCCURRENCE | Incidence in lens and non lens wearers is limited to few individuals per 10,000 wearers (4 to 5 events with daily wear; 20 to 21 with low-Dk extended wear) | Rare in non-lens wearers; 25 times more frequent with daily wear in comparison to MK; 50 times more frequent with extended wear in comparison to MK (CCLRU/LVPEI data) |
| SYMPTOMS | Moderate to severe pain of rapid onset, severe redness ('meaty' appearance), decreased visual acuity if the lesion is on the visual axis, discharge (mucopurulent), tearing, photophobia, puffiness of lids | Ranges from moderate to severe pain, foreign body sensation, irritation to asymptomatic, moderate to severe redness, tearing to asymptomatic |
| SIGNS Infiltrate |
||
| Size | Commonly >1mm, can appear as multiple focal infiltrates | Usually small, single, circular, focal infiltrate (up to 2mm) |
| Shape | Any shape; commonly irregular | Circular, well-circumscribed |
| Location | Mainly central or paracentral, sometimes peripheral | Peripheral or midperipheral |
| Depth | Anterior to mid-stroma, may involve entire depth | Anterior stroma (subepithelial) |
| Surrounding Cornea | Involved, ranges from edema with diffuse infiltrates to satellite lesions or ring infiltrate | Diffuse infiltrates limited to anterior stroma |
| Overlying Epithelium | Full thickness loss (when active) | Full thickness loss (when active) |
| Endothelial Involvement | None to endothelial dusting with cells, keratic precipitates/plaques | None |
| Anterior Chamber Reaction | Common, ranging from flare to hypopyon | Only if severe; flare and cells |
| Lid Edema | Usual | Rare |
| Bulbar and Limbal Redness | Severe | Moderate, localized |
| Unilateral/Bilateral | Usually unilateral | Usually unilateral |
| ETIOLOGY | Microbial invasion and infection (bacteria and fungi) | Toxins released by Staphylococcus aureus colonizing the contact lens surface, bacteria not found on scraping or biopsy |
| RISK FACTORS | Poor lens hygiene, overnight lens wear, immunocompromised states, swimming | Overnight contact lens wear, lens material interaction with corneal surface |
| COURSE & MANAGEMENT |
|
|
| * CCLRU / LVPEI guide, adapted from Stapleton F. et al. Risk factors with contact lens suppurative keratitis. CLAO Journal 1993; 19:204-10 and Aasuri M et al. Differential diagnosis of microbial keratitis and contact lens-induced peripheral ulcer. Eye & Contact Lens 2002;29 (IS):S60-62. | ||
|
TABLE 3 Bacterial species isolated from lens-related MK. |
|
| GRAM-NEGATIVE BACILLI (RODS) Enterobacter aerogenes Escherichia coli Hemophilus influenzae Klebsiella pneumoniae Klebsiella spp. Proteus spp. Pseudomonas aeruginosa Pseudomonas spp. Serratia marcescens Serratia spp. GRAM-NEGATIVE COCCOBACILLI |
GRAM-NEGATIVE COCCI Neisseria gonorrhoeae Neisseria meningitidis GRAM-POSITIVE BACILLI GRAM-POSITIVE COCCI |
Dr. Edwards is a research optometrist and doctoral student at the Vision Cooperative Research Centre (CRC) and at the Cornea and Contact Lens Research Unit, School of Optometry and Vision Science, University of New South Wales (UNSW).
Mr. Brian is program director of Vision Care Delivery at the Vision CRC.
Dr. Stretton is a science writer at the Vision CRC.
Dr. Stapleton is director of Post-Graduate Research at the Vision CRC and is senior lecturer at the CCLRU, School of Optometry and Vision Science, UNSW.
Dr. Willcox is associate professor at the Cornea and Contact Lens Research Unit, School of Optometry and Vision Science, UNSW.
Dr. Sankaridurg is program director of Myopia at the Vision CRC.
Dr. Sweeney is an associate professor, deputy CEO and executive director of Research at the Vision CRC and the Cornea and Contact Lens Research Unit, School of Optometry and Vision Science, UNSW.
Dr. Holden is a professor at the Cornea and Contact Lens Research Unit, School of Optometry and Vision Science, UNSW and CEO at the Vision CRC.