CL DRY EYE MANAGEMENT
Managing
Contact Lens-related Dry Eye
Once
you've diagnosed lens-related dry eye and its etiology, you can take steps toward
preventing dropout.
By Jerry R. Paugh, OD, PhD
Contact lens-related discomfort remains a significant problem in contact lens practice. In one survey (Pritchard, 1999) involving practices in Quebec, Canada, investigators found that 34 percent of 1,444 patients discontinued wear, with 12 percent becoming permanent dropouts. Interestingly, of the 488 initial dropouts, nearly half cited discomfort/irritation and nine percent cited dry eye as the reasons for discontinuing contact lens wear. Conversely, 23 percent of patients who resumed wear cited resolution of discomfort as the reason for resuming wear. Thus, managing discomfort and lens dryness continues to be an important goal to practitioners and industry alike, which explains the plethora of new products and approaches designed to ameliorate the problem.
I'll summarize what's currently known about contact lens-related dry eye, and I'll provide an overview of management approaches. Key issues include the prevalence of dry eye discomfort, tear film differences between tolerant and intolerant patients, the nature of dry eye disease among wearers and the efficacy of various approaches to managing contact lens discomfort.
Prevalence of Lens Dryness
How common are dry eye and discomfort symptom reports among contact lens wearers? Nichols et al (2005) conducted a recent investigation of symptoms in a sample of patients that included clinical emmetropes, contact lens wearers and spectacle wearers presenting for routine ophthalmic care. Of 893 responses, 52 percent of lens wearers reported dry eye disease, followed by 24 percent for spectacle wearers and seven percent for clinical emmetropes. Moreover, the percentage of patients who reported increasing frequency of dryness (from never to occasionally or frequently) was greater for contact lens wearers than for the spectacle or emmetropic groups. In a similar study, Guillon and co-workers (2002) found that 43 percent of contact lens wearers reported dry eye symptoms compared to 15 percent of non-lens wearers.
From these studies and others, considerable evidence exists that contact lenses induce dry eye symptoms in a significant number of wearers. Although clinicians consider contact lenses to be an etiologic factor in the development of dry eye (Lemp, 1995), much remains unanswered as to why symptoms develop in lens wearers.
|
|
|
|
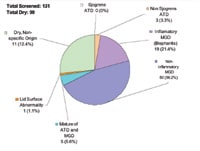
Figure
1: Classification of dry eye sub-types to date at SCCO. |
|
Clinical and Tear Composition Characteristics of Intolerant Wearers
Glasson and co-workers (2002, 2003) examined tear compositional differences and clinical tear parameters (non-invasive breakup time and tear volume) in two studies of tolerant and intolerant patients. They examined lipid and lipocalin in tear film (2002) and also proteins (2003). They defined intolerant patients as those who discontinued soft lens wear because of dryness symptoms during the first six hours of wear.
Although they found no differences in lacrimal gland secretory proteins (lactoferrin and lysozyme) in the 2003 study, the 2002 results suggested that intolerant subjects had more degraded lipid compounds, lipocalin and lipid enzyme activity compared to tolerant subjects. Interestingly, the lipid layer appearance demonstrated no differences between the subject groups in either study.
Also using lipid analysis, Guillon and co-workers (2002) demonstrated that greater levels of tear cholesterol esters were associated with a less stable lipid layer and increased discomfort symptoms. Although further study is needed, it appears that identifiable differences exist among wearers and that they may involve the lipid components of the tear film.
A key outcome of the work of Glasson et al (2003) was that they found two easily measured tear parameters, tear volume (via phenol red test and tear meniscus height) and non-invasive breakup time (NIBUT), to be significantly reduced in the intolerant wearers. Similarly, in a European study (Andres, 1987), 85 percent of intolerant lens wearers had fluorescein breakup times of <10 seconds. This suggests that measuring these clinical parameters, perhaps as part of a dry eye screening, may help identify patients who are likely to experience lens wear difficulties.
Discomfort Due to Dry Eye
It's well known that contact lenses induce major changes in the tear film including increased evaporation (Tomlinson and Cedarstaff, 1982), decreased stability (Faber, 1991) and decreased thickness (Nichols, 2005). Although healthy, normal patients seem capable of achieving good comfort with lens wear in spite of these changes, a significant number of patients find lens wear intolerable. The causative factor might be underlying dry eye disease, particularly in our aging population.
|
|
|
|
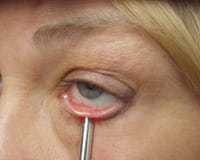
Figure 2. Meibomian
expression demonstrating a Grade 2 (thickened and opaque) secretion. |
Clinic-based Dry Eye Sample Characteristics
For several years, the Center for Vision Research at Southern California College of Optometry has undertaken studies related to dry eye diagnosis, product evaluation and applied research into areas such as artificial tear residence time and effect on vision. To evaluate some newer dry eye diagnostic techniques, an ongoing screening study is underway that shows the general characteristics of a clinic-based dry eye sample and of patients referred for lens intolerance that we believe is related to a fundamental dry eye condition.
In terms of diagnosis, we classified subjects by dry eye sub-type, as outlined by Pflugfelder et al (1998). The major categories are:
-
Sjögren's aqueous tear deficiency (SATD)
-
Non-Sjögren's aqueous tear deficiency (NSATD)
-
Blepharitis (or inflammatory) meibomian gland dysfunction (BLEPH)
-
Atrophic (or non-inflammatory) meibomian gland dysfunction (AMGD)
-
Mixture of aqueous tear deficiency and meibomian gland dysfunction
Figure 1 presents the current status of the screened individuals. One of the most striking findings is that overall, approximately 75 percent have dry eye related to meibomian gland dysfunction, either of the inflammatory or non-inflammatory type.
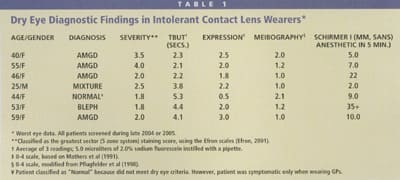
Several evaluations for the dry eye screening study involved referrals from the contact lens service, which referred the patients because they were having significant difficulty in successfully wearing their lenses. Table 1 provides characteristics of a small, recent sample of these subjects. It appears that, as with the larger dry eye group, the majority of intolerant contact lens patients (six out of seven) also have dry eye related primarily to meibomian gland disease. This reinforces the idea that we must, as clinicians, evaluate the meibomian glands as part of a broader dry eye workup in intolerant patients.
Diagnosing Contact Lens-related Dry Eye
Before managing dry eye syndrome, we must establish an adequate clinical testing system and attempt to assign a dry eye sub-type when possible. From our experience to date, it seems reasonable to use the sub-type classification scheme of Pflugfelder et al (1998). The main goal is to differentiate aqueous deficiency (by use of Schirmer, fluorescein clearance test, and/or tear meniscus height) from meibomian gland disease. Following are the minimal recommended tests, in order from least invasive to most invasive:
-
Tear meniscus height using a reticule eyepiece — provides tear volume estimate.
-
Biomicroscopy, searching for facial flush area injection, eyelid injection and lash loss — all components of inflammatory MGD.
-
Fluorescein or non-invasive breakup time — average three measurements, allowing 30 seconds rest between consecutive measurements.
-
Corneal staining using fluorescein and yellow barrier filter — recommended five-zone N.E.I. system (Lemp 1995) and 0 to 4 scale.
-
Rose bengal or lissamine green conjunctival staining — stains mucus–free areas.
-
Meibomian gland expression (Figure 2) — 0 to 4 scale as in Table 1.
-
Meibography using clinical transilluminator (Figures 3 and 4) — both lower lids, scale 0 to 4 as in Table 1.
-
Schirmer I test — five minutes, without anesthetic.
Instruct patients to not wear their contact lenses for at least two days before dry eye evaluation to allow any lens-related staining to clear. Also, patients should use no lubricant drops on the day of the dry eye workup.
Diagnostic Criteria It's challenging to develop dry eye cut-off criteria for the various tests because of the diversity of reported study approaches and dry eye populations. Many investigators have adopted the criteria of the N.E.I./Industry workshop (Lemp 1995). The principal criteria for diagnosing dry eye include symptoms, tear instability, evidence of ocular surface damage and increased tear osmolality. With the exception of osmolality, you can easily measure these parameters clinically. The criteria we use at our clinic are based on the workshop principles, but because of recent literature reports, we've tempered them more conservatively as follows:
-
Symptomatology — modified Schein score >7/24 possible (Paugh 2003)
-
TBUT < seven seconds
-
Corneal fluorescein or conjunctival rose bengal staining:
Cornea — five-zone system (0 to 4 scale); staining > 4/20 possible for all five zones
Conjunctiva — six-zone system (0 to 4 scale); > 4/24 possible for six zones
-
Schirmer I sans anesthesia — < 5mm in five minutes
|
|
|
|
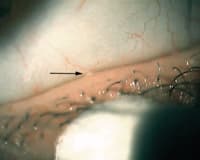
Figure 3.Clinical meibography using transilluminator to evert the lower lid. |
|
|
|
|
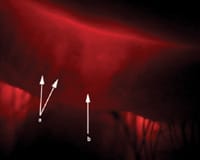
Figure 4. Meibography demonstrating an area of normal glands (letter a) and gland dropout (letter b). |
Treating Dry Eye Conditions
You can successfully implement several approaches for aqueous deficiency, using more aggressive measures for greater severity. Aqueous deficiency treatments include conservation of existing tears (as with punctal plugs — first temporary plugs, then silicone plugs if effective), and replacement of missing aqueous (artificial tears). If the deficiency is severe, add simultaneous treatment with an anti-inflammatory such as Restasis (Allergan) or a soft steroid (such as Lotemax, Bausch & Lomb). If you use a steroid, take care to first evaluate the optic cup and measure intraocular pressure (IOP) in case of steroid response. Thick nighttime lubricants or gels can assist healing of epithelial damage during sleep.
The ocular surface dwell time is influenced by viscosity and possibly whether the polymer in the formulation is muco-adhesive. Generally, residence time increases as the viscosity changes from a thin, watery preparation, to higher-viscosity gels and ointments. Preliminary data in our laboratory suggest that higher-viscosity formulations (Systane [Alcon] and Refresh Liquigel [Allergan]) may last approximately twice as long as saline in the eyes of dry eye subjects (Paugh 2004).
Managing MGD, whether inflammatory or non-inflammatory, has at its center warm compresses and lid massage. Paugh et al (1990) examined warm compress/lid massage therapy in a group of 21 problematic soft lens wearers whose symptoms didn't seem related to care regimen or lens fit problems. The researchers left one eye untreated and treated the fellow eye bid with warm compress therapy. Under masked conditions, an investigator measured fluorescein breakup time (TBUT) and found that the treated eyes had increased approximately four seconds compared to no change for the untreated fellow eyes. Four seconds is clinically significant and correlated with increased comfort of the treated eye.
Severe inflammatory MGD is relatively rare in contact lens practice, but may be the most challenging condition when attempting to rehabilitate such patients to resume contact lens wear. We've seen a handful of such cases over the past few years that demonstrated several common features. All eventually resumed contact lens wear.
All patients had moderate to severe (grade 3 to 4 on a 0 to 4 scale) corneal staining, greatly reduced TBUT (< five seconds) and moderate to severe meibomian gland atrophy (> 50 percent of glands missing, with variable presentation of meibomian cysts). In all cases, successful therapy included two to four months of warm compresses and lid massage; topical (erythromycin ointment qhs) and systemic (minocycline or doxycycline bid, 100mg/day) antibiotics; and topical steroid application (Lotemax qid, monitor IOP and optic cup, then taper).
While warm compress with lid massage remains the primary approach to MGD, other management options have become available. These include lipid replacements (Soothe [Alimera Sciences] or Refresh Endura [Allergan]) and possibly systemic ingestion of omega-3 fatty acids. We have limited clinical data on efficacy for this latter approach, although the mechanism may be an anti-inflammatory effect on the ocular surface (Barabino 2003).
Lens and Care Regimens
Although relatively recent, a growing effort is underway by both lens and solution manufacturers to remedy the discomfort/dryness issues related to lens wear. While much remains unknown, several studies have looked at the impact of these efforts in improving lens wearing comfort and in vivo performance.
Fonn and co-workers (1999, 2003) examined comfort over seven hours in symptomatic and asymptomatic wearers with several lenses (omafilcon A, etafilcon A, nefilcon A and lotrafilcon A). They found that comfort decreased significantly and equally with all materials over time only for the symptomatic wearers. These findings suggest that lens comfort is more patient-specific rather than material-specific, at least with these materials.
Thai and co-workers (2004) also examined aspects of biocompatibility of several lens materials, using measures of tear physiology. They studied polymacon, omafilcon A, phemfilcon A, balafilcon A and etafilcon A in successful hydrogel lens wearers. They found no significant differences among lens materials, with the exception of improved pre-lens tear film structure for omafilcon A.
Another study by Thai and co-workers (2002) examined the effect of a regimen adjunct (hydroxypropylmethylcellulose [HPMC] in AMO's Complete Comfort formulation) on clinical measures of tear physiology in normal wearers. They found no differences in tear evaporation or comfort, but significant differences for tear film structure (measured with interference images) and pre-lens tear stability (measured non-invasively) in favor of the HPMC formulation.
Taken together, there appear to be subtle yet measurable improvements in patient acceptance and objective indicators when using certain lens materials and care regimens. These options are worth exploring for patients who suffer from dryness issues.
Where Do We Go From Here?
Dry eye symptoms are frequent in contact lens wear and may induce significant numbers of patients to discontinue wear. Easily measured clinical parameters such as tear stability and volume are associated with contact lens intolerance and may point to a patient-specific origin. Dry eye in general, and also that associated with contact lens intolerance, appears biased toward meibomian gland disease.
Therefore, diagnosis of dry eye by sub-type, in conjunction with appropriate management, appears vital to allowing these patients to continue lens wear. We have limited, but definite evidence that certain lens materials (omafilcon A) and possibly "moisturizing" care regimens may provide product-related tools to manage contact lens-related dryness. Additional clinical research is needed, using in vivo tear measures, to fully characterize recent material and regimen advances.
To obtain references for this article, please visit http://www.clspectrum.com/references.asp and click on document #118.