REWETTERS
Chemical Properties of Contact Lens Rewetters
A review of hyaluronic acid as a contemporary
ingredient in contact lens rewetters.

Figures from the Global Industry Analysts February 2004 report indicate that the number of contact lens dropouts was estimated at almost 2 million in 2001. Discomfort — especially because of dryness — was one of the major problems that compelled consumers to stop using lenses.
Contact lens wearers may decide to wear contact
lenses less frequently or to totally discontinue lens wear because of dryness symptoms
(Table 1). To provide contact lens wearers with significant relief from the symptoms
of ocular discomfort and chronic irritation, eyecare manufacturers have introduced
a variety of rewetting/
comfort formulations for use during contact lens wear.
|
TABLE 1 |
|
Lens-induced
Dry Eye Symptoms
•
Dryness |
I had an opportunity to serve as a masked clinical investigator during an FDA pre-approval study sponsored by Advanced Medical Optics, Inc. (AMO) to evaluate three potential rewetting agents, all containing hyaluronic acid (HA) as a key ingredient. Blink Contacts (AMO) rewetting drops, with a 0.15% HA content, emerged as the superior formulation. Throughout the study, I found that drops containing HA stabilized the tear film, increased TBUT and improved comfort and contact lens wear tolerance.
This article will evaluate HA as well as other ingredients of rewetter formulations to provide you with a wider comprehension of rewetting eye drops in the market today.
Rewetter Ingredients
Table 2 delineates the composition of currently available rewetting products. Some of these formulations were initially marketed as an artificial tear, then manufacturers eventually developed the add-on capability of contact lens rewetting. Some are considered companion products, meaning they were branded to accompany a multipurpose solution. Other formulations evolved from these companion products, with additional ingredients intended to moisturize, clean or lubricate, rather than merely to rewet the contact lens.
To adequately soothe the ocular surface, modern-day rewetting drops typically contain a wetting ingredient (such as a surfactant, ocular demulcent or sodium hyaluronate), a preservative system, a buffer and an electrolyte system.
Wetting Agents
|
|
|
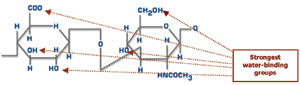
Figure 1. Structure of hyaluronic acid (HA). |
Surfactants, or surface active agents, contain both hydrophobic and hydrophilic moities, enabling them to bind to both protein and water. Because of their water-binding property, surfactants are able to enhance the wettability of rewetting formulations. Lens care manufacturers often use surfactants as detergents for cleaning and removing surface debris. While all surfactants function as cleaners, particularly against proteins, these agents also act as wetting agents. Examples of surfactant-containing lens lubricants are Opti-free Replenish rewetting drops (same formulation as Clerz Plus lens drops; Alcon Laboratories), which contain a variety of surface active agents, and Complete Blink-N-Clean lens drops (AMO), which use tyloxapol to clear away ocular precipitates that can cause irritation and discomfort.
Tetronic 1304 Surfactant helps lenses retain moisture and also helps shield the lens from future protein build-up. There are many different Tetronic designs; the primary function of Tetronic 1304 as listed by BASF is a wetting agent. In a recent study (Subbaraman et al, 2006) instillation of a rewetting drop containing Tetronic 1304 resulted in adsorption of the surface active agent to silicone hydrogel lenses, which increased the wettability of the lens surfaces. This is the only published study examining the use of rewetting drops in silicone hydrogel lens wearers. Tetronic 1304 is the surfactant in Opti-Free Express rewetting drops and Opti-Free Replenish rewetting drops. Opti-Free Replenish also contains polyethylene glycol-11 lauryl ether carboxylic acid (also called RLM-100), a surfactant designed to remove protein and lipids.
Ocular Demulcents
In 1980, the FDA created a monograph of over-the-counter (OTC) ophthalmic drug products that are recognized as generally safe and effective. The monograph, which is part of an ongoing FDA review of drug products, defines an ocular demulcent as "an agent, usually a water-soluble polymer, which is applied topically to the eye to protect and lubricate mucous membrane surfaces and relieve dryness and irritation." The originally listed FDA-approved ocular demulcents carboxymethylcellulose (CMC), hydroxypropyl methylcellulose (HPMC), glycerin and povidone are still used today in a number of rewetter formulations.
|
TBUT and Contact Lens Wear |
|
A widely used technique of measuring TBUT is by slit
lamp observation of the tear film after fluorescein instillation. Scientific studies
have demonstrated that contact lens wear causes alterations in the normal tear film,
such as abnormalities in tear osmolarity, tear protein composition and TBUT. Glasson
et al (2003) found that contact lens-intolerant subjects had a shorter TBUT and
a greater number of symptoms associated with ocular surface discomfort compared
to lens-tolerant subjects. Thus, a formulation that prolongs TBUT even for just a few seconds may bridge the gap between breakup time and blink time, achieving improvement in ocular surface protection. |
Carboxymethylcellulose (CMC) is a synthetic, water-soluble cellulose derivative with excellent water-binding and bio-adhesive properties. CMC-based solutions can protect the eye during LASIK surgery and can accelerate ocular surface recovery by improving post-operative tear film stability and ocular surface staining. A study (Grene et al, 1992) of 56 dry eye patients demonstrated that patients treated with unpreserved CMC-based artificial tears showed improved fluorescein staining, symptoms and cytology grades. CMC can also reduce the bioactivity of disinfecting agents in multipurpose solutions (MPSs) by chemically complexing the active agent, thus protecting the eye from ocular surface insult. CMC is an OTC ophthalmic demulcent when used at a concentration of 0.2% to 2.5%. Refresh Contacts comfort drops (Allergan, Inc.) contains 0.5% CMC.
Povidone is a water-soluble polymer found in ReNu MultiPlus rewetting drops (Bausch & Lomb). Patients administered polyvidone-containing tear substitutes experienced improved dry eye symptoms while exhibiting high tolerability for the compound. Povidone is an ophthalmic demulcent when used at a concentration of 0.1% to 2%.
Hydroxypropyl methylcellulose (HPMC) Toda et al (1996) showed that preservative-free 0.5% hydroxypropyl methylcellulose (HPMC) improved dry eye symptoms and decreased ocular surface damage in two groups of dry eye patients (those with Sjögren's syndrome and those without), possibly because of extended retention time and mechanical protection of epithelial cells. Because of its high viscosity, HPMC remains on the ocular surface longer than aqueous or polyvinyl alcohol (PVA) solutions do, with a mean retention time of 5.1 minutes. In a study (Simmons et al, 2001) of 147 subjects wearing hydrogel lenses, patient satisfaction and contact lens wearing time improved significantly when patients used an MPS containing HPMC. HPMC is an OTC ophthalmic demulcent when used at a concentration of 0.2% to 0.5%. Visine for Contacts rewetting drops (Pfizer, Inc.) contains HPMC.
Glycerin, the other demulcent found in Visine for Contacts rewetting drops, is a trihydric alcohol with high water-binding properties. Glycerin is highly viscous and not likely to dry and harden by exposure to air. It's also hygroscopic, meaning it absorbs water from the air, helping to retain surface moisture. Glycerin can also serve as a tonicity-adjusting agent. Glycerin functions as an ophthalmic demulcent at concentrations of 0.2% to 1%.
Preservatives
|
|
|
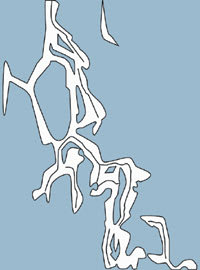
Figure 2. HA under low shear force (between blinks). |
Most eyecare products contain preservatives to ward off bacterial contamination. For patients who use drops frequently or regularly, preservative free or "disappearing preservative" drops are often recommended to prevent any preservative cytotoxic effects.
Sodium perborate, found in Aquify (CIBA Vision) eye drops, is considered a transient preservative because it transforms into water (which stays in the eye) and oxygen (which is dispelled into the air) after instillation into the eye. When combined with water, it converts into hydrogen peroxide, which makes an effective anti-microbial agent.
Stabilized oxychloro complex (SOC) is an ophthalmic preservative at low concentrations (0.005% weight per volume). SOC is found in blink Contacts eye drops as OcuPure preservative and in Refresh Contacts eye drops as Purite preservative. Way et al (2001) found that SOC was the least disruptive to the cellular integrity of epithelial cells, compared to benzalkonium chloride (BAK), cetrimide, phenylmercury borate, Polyquad (Alcon) and perborate. SOC is also considered a 'disappearing' preservative because it breaks down into sodium chloride and water when exposed to light, rendering the solution virtually preservative-free upon instillation.
Edetate disodium (EDTA) preservative chelates calcium and magnesium ions, which are responsible for the cross-linking of polysaccharides in microbial cell membranes. EDTA is found in Opti-Free Express rewetting drops and in ReNu MultiPlus eye drops.
Polyquaternium-1 (Polyquad) preservative is used as a disinfecting agent at 0.001% concentration. The large molecular size of Polyquad prevents it from penetrating into hydrogel contact lenses. Polyquad is found in Opti-Free Express rewetting drops and Opti-Free Replenish rewetting drops.
Sorbic acid (potassium sorbate) is an unsaturated carboxylic acid with fungistatic and limited anti-microbial properties. At certain concentrations, the products of sorbic acid oxidation are known to discolor some hydrophilic contact lenses.
Electrolytes
The electrolyte content of human tears generally resembles that of blood plasma, but with some differences. All of the rewetting drops in Table 2 contain sodium and chloride. Experiments using rabbit corneas demonstrated that the corneal epithelium is best maintained with a buffered solution containing potassium, calcium, magnesium, phosphate and bicarbonate, in addition to sodium and chloride. Calcium, magnesium, potassium, sodium and chloride are all found in blink Contacts eye drops and Refresh Contacts eye drops, and Renu MultiPlus eye drops additionally has potassium.
|
|
|
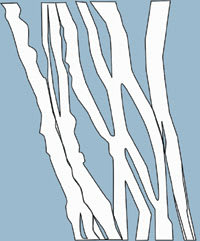
Figure
3. HA under high shear force (during blinking).
|
Buffers
Buffers maintain the ophthalmic solution's pH within the buffering capacity of the eye. This means the buffer resists unwanted pH changes and in the process, helps prevent the ophthalmic solution's precipitation and deterioration.
Sodium phosphate, found in Aquify comfort drops, is the most physiologic of all the common buffers, as it's a natural buffer occurring in tears.
Boric acid is an anti-fungal agent and preservative-enhancing additive, and therefore minimizes the use of increased concentrations of preservative in the ophthalmic solution. Boric acid coupled with sodium borate decahydrate (borax) acts as a good buffer system that has anti-microbial activities, as boron (acting with oxygen and salt) helps disinfect by killing bacteria and fungi. Boric acid and sodium borate decahydrate comprise the buffer system found in blink Contacts eye drops and Refresh Contacts. Opti-Free Replenish, ReNu MultiPlus and Visine for Contacts rewetting drops also use the borate buffer system.
Citrate is a buffering and chelating agent as well as a lens-cleaning component of MPSs and is found in Opti-Free Replenish and Opti-Free Express rewetters. This compound works by a unique ionic displacement mechanism that allows it to displace protein, the primary component of lens deposits, during the cleaning procedure. Hong et al (1994) found that it passively removed protein from Group IV lenses.
Next-Generation Comfort Ingredient
Each formulation in Table 2 contains what is referred to as a 'comfort ingredient,' the main component responsible for the product's ability to comfort or soothe the eye. The ideal comfort ingredient should display high water-binding and viscoelastic properties, enabling it to bind more water, to spread quickly between blinks and to remain longer on the cornea without causing visual blurring. A higher molecular weight in comfort ingredients enables them to bind more water and support a thicker tear film. Also, the ideal comfort ingredient should be able to stabilize the tear film during contact lens wear, resulting in lens surface wetting and the reduction or elimination of ocular dryness and discomfort.
Hyaluronic acid (HA) is a naturally-occurring, high-molecular-weight, viscoelastic component of synovial joints, extracellular matrix, vitreous humor, aqueous humor and skin. Because of its viscoelastic properties, HA can function as a shock-absorbing fluid in joints and as an ocular lubricant. HA is an efficacious therapeutic agent for dry eyes. Mengher et al (1986) demonstrated that a concentration of 0.1% sodium hyaluronate in preservative-free eye drops can increase tear film stability and alleviate dry eye symptoms, and concentrations of 0.1% and 0.3% can delay TBUT. Manufacturers have recently incorporated HA as a comfort ingredient into the newest line-up of rewetter drops in the eyecare market: Aquify comfort drops, containing 0.1% HA, and blink Contacts eye drops, containing 0.15% HA.
HA is used in ophthalmic practice to:
• Protect the corneal endothelium and prevent anterior chamber collapse during intraocular surgery.
• Treat dry eyes because of its water retentive properties.
In addition, HA in vivo and in vitro inhibits the formation of free radicals, which are partly to blame for cell damage arising during cataract surgery.
Compared to other ocular demulcents, 0.2% sodium hyaluronate has a longer mean half-life in artificial tears solutions of 321 seconds on the ocular surface (0.3% HPMC's half-life is 44 seconds while 1.4% PVA's is 39 seconds). When compared to tear substitute preparations containing PVA, hydroxymethylcellulose and HPMC, sodium hyaluronate (Healon 0.1% ophthalmic viscosurgical device) best stabilized the precorneal tear film.
How does hyaluronic acid work?
The chemical structure of HA accounts for its ability to function as an effective
lubricant in the musculoskeletal system and as a mechanical cushion in the eye.
Its structure resembles that of a sponge made of polysaccharide chains with water
trapped between its molecules (Figure 1).

High-viscosity agents are effective for binding water to the eye, but have the following disadvantages:
• Formation of uncomfortable crusty residues.
• Their inability to spread evenly on the eye surface, causing blurring.
• Their tendency to increase friction during high shear rates, resulting in decreased lubrication.
HA's unique physical properties enable it to maintain high viscosity without causing residue formation, blurring and friction. How does HA do this?
HA is a pseudoplastic molecule, meaning it has high viscosity at low shear rates and high elasticity at high shear rates. HA exists in a random coil configuration that is poly-anionic at physiological pH. Under low shear force (when the eye is open), HA molecules are randomly tangled and highly viscous (Figure 2). During this time, TBUT slows down and tear evaporation and drainage are delayed.
Under high shear force (when the eye blinks), HA molecules align, thereby allowing the flow of water between the chains, making the compound less viscous (Figure 3). At this point, HA spreads easily and evenly over the ocular surface, providing effective lubrication.
A clinical study of contact lens rewetters by Kao et al (2004) showed that blink Contacts eye drops prolonged TBUT time in contact lens wearers by 3.1 seconds over baseline after 30 days of use. The study also revealed a direct correlation between HA concentration and TBUT — the 0.15% concentration yielded an increase of 3.1 seconds in TBUT, while 0.1% resulted in an increase of less than two seconds. Prolonged TBUT time is associated with improved end-of-day comfort scores from baseline to day 30. Blink Contacts contains a 0.15% concentration of HA.
Summary
Ultimately, a successful contact lens rewetter/lubricant relieves ocular discomfort and prolongs lens wearing time. If a rewetting product entails frequent instillation to manage symptoms of discomfort and dryness, then its utility is limited. The new generation of HA-containing drops may be able to provide longer-lasting relief from dryness without the inconvenience of repeatedly applying the solution to the eye.
For references, please visit www.clspectrum.com/references.asp and click on document #125.
Dr. Szczotka-Flynn is an associate professor at Case Western Reserve University Dept. of Ophthalmology and is director of the Contact Lens Service at University Hospitals of Cleveland.