SCLERAL LENSES
Fitting
Scleral Lenses
Scleral
lenses can offer excellent comfort and vision for both compromised and normal corneas.

That necessity is sometimes the mother of invention is born out in how modern scleral lenses developed. Seeking a protective system to deliver medication to eyes ravaged by inflammation, Dr. Perry Rosenthal seized upon the work of Australian optometrist Don Ezekiel, who in 1983 reported success with an air-ventilated silicone acrylate scleral lens. Their pioneering work began in the late 1980s and led to the development of the Boston Scleral Lens (Dk 100, Boston Foundation for Sight [501c3]).
Utilizing spline curves, diameters between 15.0mm to 24.0mm and a unique tear channel system, the Boston Scleral Lens is like a liquid corneal bandage to treat severe conditions such as severe dry eyes, ocular cicatricial pemphigoid, Stevens-Johnson syndrome, persistent epithelial defects, keratoconus, pellucid marginal degeneration and penetrating keratoplasty. The lens is supported almost entirely by the sclera, and the central optic zone (12mm) is designed to completely vault the cornea. The Boston Scleral Lens provides a protective shell and therapeutic reservoir, and it has improved vision and symptoms in patients for whom nothing else has worked.
In addition to the Boston Scleral Lens, other scleral lenses of similar designs are also available. These include the Jupiter Mini-scleral and Scleral (Innovations in Sight), the Macrolens Elite and O series (C&H Contacts) and the Semi-Scleral (Abba) lens. These designs are available in various diameters from just beyond the limbus (corneal-scleral) to sizes well onto the sclera (18.0mm to 24.0mm).
The following cases illustrate the therapeutic uses of two of these scleral lens designs.
|
|
|
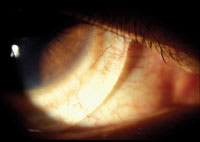
Figure 1. Apical clearance with the Boston Scleral lens. |
Case 1
A 21-year-old African-American male who had moderate keratoconus was referred for contact lens fitting because of corneal apical staining and early apical scarring. The patient was wearing GP lenses that had been fit elsewhere. Fluorescein analysis demonstrated a large apical-bearing zone of 5mm to 6mm in diameter. Entering aided acuities were OD 20/25 and OS 20/30, which were two to three lines better than best-correctable spectacle acuity because of the irregular astigmatism created by the ectasia. Corneal topography including axial curvature and direct elevation mapping demonstrated a central cone OD and a slightly nasally eccentric cone OS. Simulated keratometry yielded values of OD 48.96/59.15 @ 128 and OS 38.26/52.22 @ 079. We advised the patient to discontinue lens use for two weeks and to return for lens fitting.
After two weeks of not wearing the lenses, we fit the patient into Rose K (Blanchard Contact Lens, Inc.) GP lenses. We informed him that corneal changes would likely occur over the next few weeks because of the molding effects from his previous lenses. Over the next four months the corneas continued to remold, with each eye increasing approximately 0.50D in steepness each month. With each change, we made a corresponding change in the lenses to stay ahead of the cone and to maintain feather touch fit. The peripheral clearance required adjustments to prevent seal off and to maintain adequate movement.
During the next five years, the patient's keratoconus continued to progress bilaterally, requiring lens changes and peripheral curve adjustments every six months. By 2003, the patient's contact lens prescription was OD base curve 4.38mm (77.05D), diameter 9.90mm, maximum peripheral flattening, power of –24.75D, Boston XO and OS base curve 4.60mm (73.37D), diameter 9.90mm, maximum peripheral flattening, power of –24.00D, Boston XO. At this point apical bearing was apparent, yet steepening the lens made it difficult to fabricate appropriate peripheral curves and powers.
We suggested alternatives of a piggyback lens system, a full-thickness corneal graft or scleral contact lens fitting. The patient opted to be fit with the Boston Scleral Lens, so we referred him to the Boston Foundation for Sight.
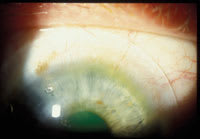
On follow-up examination after the patient received the lenses, slit lamp examination demonstrated a well-centered lens with 1mm to 2mm of apical clearance (Figure 1). The lens showed no movement at all and we found significant blanching of perilimbal conjunctival vessels. The blanching pattern exhibited bands of blanching and engorgement, denoting decreased conjunctival capillary perfusion due to compression (Figure 2). We instilled sodium fluorescein three times and found no evidence of tear flow under the lens after 15 to 30 minutes.
The patient returned to the Boston Foundation for Sight for lens fit adjustments. On subsequent examination, flattening of the peripheral curves demonstrated an improved bearing relationship between the lens and the scleral haptic. Decreased blanching of perilimbal conjunctival vessels denoted minimal bearing peripherally. The lenses continued to demonstrate little to no movement and an apical clearance pattern was still evident. Most importantly, the lenses demonstrated adequate tear exchange by the observance of fluorescein under the lens some 15 minutes after instillation. The Boston Scleral lens specifically avoids fenestration through the use of channels designed to prevent seal off while also preventing bubble formation. Desiccation is a potential significant cause of treatment failure in inflammatory disease and must be avoided in cases of severe pathology. The patient's visual acuity continued to hover in the 20/25 to 20/30 range, which he deemed acceptable.
|
|
|
Figure 2. Pronounced blanching and engorgement from poor-fitting scleral lens. |
Over the past year the patient's cornea has well tolerated a daily-wear schedule, with no increase in apical staining or scarring and no evidence of corneal edema or anterior chamber reaction. Only recently has the patient's right eye started to demonstrate negative tear flow under the lens again. He will return to Boston Foundation for Sight for follow up.
Case 2
A 30-year-old white male presented with a history of keratoconus OD and a full-thickness corneal graft OS, performed three years prior. He presented to be fit with a contact lens OS. His right eye had previously been fit with a lens that yielded excellent visual acuity of 20/25. He wore no lens in his left eye. Best-corrected spectacle acuity in this eye was 20/40, resulting from uncorrected irregular astigmatism inherent in many corneal grafts. Biomicroscopy evaluation OD demonstrated slight apical thinning, with minimal to no other signs of keratoconus. The GP lens OD centered well, with an acceptable three-point touch, good tear exchange and good movement.
The left eye demonstrated a crystal clear graft with normal pachymetry in the four major quadrants. Keratometry revealed the expected mild distortion of mires, with readings of OD 48.37 @ 057/ 50.00 @ 148 and OS 42.75 @ 175/43.00 @ 086. Placido disc topography revealed an inferiorly displaced cone OD with simulated Ks of OD 51.92/ 47.46 and OS 44.00/41.56. The corneal graft was oblate in appearance and, by topography, had steeper host tissue than donor tissue.
We performed a diagnostic fitting and ordered a Dyna Intralimbal Lens (Lens Dynamics) of large diameter design to increase stabilization. Parameters OS included a 10.4mm diameter, 7.34mm base curve, power of –6.75D, periphery 1.00D flatter than standard, Korb edge, Boston XO material. We instructed the patient to limit wear time and to return within one to two weeks with the lens in place so we could discern what adjustments might be needed.
Seven months later the patient returned for his first follow-up examination. He had been out of the country on an archeological dig and had been unable to respond to our attempts to schedule a follow-up appointment. Fortunately, the patient hadn't been wearing the lens because of discomfort and thus no corneal damage had occurred. Examination revealed significant inferior decentration of the lens most likely because of its excessive mass. Dyna Intralimbal lenses usually center exceptionally well, but when they don't, attempt to correct the problem by decreasing the mass.
We decided to pursue other lens designs. We made attempts to fit the patient with Rose K, Post Graft (Lens Dynamics), Aspheric (Boston), reverse geometry and Polycon II (CIBA Vision) lens designs. All were unstable, with most decentering laterally as often occurs with corneal graft patients. Bitorics were incompatible with the corneal toricity, and the patient didn't prefer countersunk and piggyback lenses.
These difficulties left only scleral GP lenses as an option. Nearly one year after initially presenting to our cornea clinic, we fit the patient with the Jupiter lens (Innovations in Sight). This lens is available to all practitioners and is manufactured in Boston XO material in a variety of custom parameters. Designed to provide good central alignment while resting on the sclera for support, you can also fit the Jupiter lens with apical clearance. As with the Boston Scleral Lens, the Jupiter lens must avoid peripheral seal off to provide adequate physiology through use of appropriate peripheral curves or fenestration. Thus, movement of the lens is desired but not absolutely necessary for success on a daily wear basis. However, it is important to avoid excessive corneal bearing and bubble formation. In addition, adequate tear exchange must occur even if movement isn't obvious.
This lens yielded excellent centration, central clearance and almost complete absence of perilimbal blanching as we'd seen with the Boston Scleral lens in Case 1 (Figure 3). The most obvious exchange occurred at the site of fenestration, which is always placed outside of the donor tissue interface, as close to perilimbal as possible, to prevent desiccation of donor tissue. Carefully monitor fenestration to ensure that significant desiccation does not occur. The patient continues to wear the lens successfully, with acuities of OD 20/25 and OS 20/20.
|
|
|
Figure 3. Minimal to no vessel compression or engorgement. |
Discussion
These two cases illustrate some of the issues that you must consider when selecting scleral contact lenses for patients who have such disorders as keratoconus and irregular astigmatism. Patients that previously were unlikely to achieve stable clear vision now can enjoy this benefit as well as improved comfort.
Scleral contact lenses have come full circle thanks to new technology that allows a lens modality once considered harmful to be beneficial. The question remains as to their future beyond therapeutic uses. Scleral lenses have a role in the treatment of persistent epithelial defects and inflammatory degenerations of the ocular adnexa. These lenses offer much, but we must modify our thinking if we are to add these lenses to our armamentarium. The following are some of the issues we must resolve before these lenses can become more widely used.
How important is lens movement? Historically, lens movement has been of paramount importance in fitting contact lenses. However, we now have clinical experience showing that, in some circumstances, obvious clinically observable lens movement may not only be unnecessary, but may also be undesirable.
For instance, movement of the Boston Scleral Lens may have a deleterious effect on the healing of persistent epithelial defects. The advantage of this lens is that it vaults the cornea and reduces cornea-lens friction as compared to the friction associated with soft lens bandages. While vaulting or clearance may reduce acuity in some cases, its benefits are very important in other cases. For example, I believe the vaulting effect was beneficial in our keratoconus patient because it subjected his already scarred corneas to less trauma. He also demonstrated no adverse corneal effects, even a year later, from a lens that doesn't move.
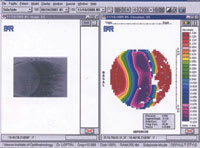
Even the Jupiter lens, which is smaller in diameter than the Boston Scleral Lens, appears to require less movement than most GP or soft lenses do. In general, the greater the tear film reservoir between the cornea and posterior lens, the less movement is required. Therefore, movement in corneal-scleral lenses may be more critical than movement with larger scleral lenses that vault the cornea. You can facilitate movement by increasing mass and fenestration. I believe placing the fenestration over the limbal region is critical. We're currently working at Mason Eye Institute (supported by Research to Prevent Blindness) with PAR corneal topography (Figure 4) to create a scleral curvature database, which may provide a more efficient method of fitting sclerals in the future.
How important is tear exchange? Stagnated tear film is associated with the potential for build up of epithelial and inflammatory cells, so it's important to carefully watch for the development of tear stagnation and post-lens debris. Radial channels allow for adequate exchange in the Boston Scleral Lens, while in the Jupiter lens, peripheral curve design and fenestration are important. Lens adherence can be problematic and must be guarded against in the smaller diameters. Christine Sindt, OD, of the University of Iowa recommends rinsing the lens with unpreserved saline just before lens application. Dr. Sindt suggests using larger diameter lenses and sagittal depth calculations when fitting these lenses.
|
|
|
Figure 4. Temporal limbus sclera viewed with PAR corneal topography. |
What about entrapment of air bubbles? Entrapment of air bubbles under scleral lenses is a relative, not an absolute, indicator of poor physiological performance. Air bubbles are ubiquitous in scleral lens fitting. Large, confluent, centrally located air bubbles are obviously not only optically debilitating, but can also cause corneal desiccation. These types of air bubbles are absolute contraindications for continued use of the lens. On the other hand, smaller-diameter bubbles may pose less of a risk. If the bubbles are tiny (no greater than 1mm to 2mm), transient, mobile and peripheral, a non-inflamed cornea may tolerate one or two air bubbles without difficulty. Inflammatory conditions, however, may be less forgiving. To discourage bubble formation, retrain patients on lens application to decrease the likelihood of air bubbles. Patients must apply the lenses while they look downward.
Any contraindications for scleral lenses? Patients whose corneas demonstrate significant edema because of reduced endothelial cell count may be poor scleral lens candidates. However, scleral lenses do present an opportunity to dramatically increase comfort and decrease apical scarring in some keratoconic and corneal graft patients.
Don't feel that you must limit scleral lenses to compromised corneas. These lenses eliminate lid-lens interactions, which are a major source of discomfort for some GP lens wearers, especially in situations where unstable dynamics occur with patients who have irregular astigmatism. You can also use scleral lenses to avoid the hypoxia induced by some piggyback lens systems and hybrid lens designs.
Dr. Loftin is a clinical assistant professor at Mason Eye Institute, Department of Ophthalmology, University of Missouri School of Medicine, Columbia, MO.
For references, please visit www.clspectrum.com/references.asp and click on document #125.