OXYGEN AND LENS WEAR
The Role of Oxygen in Successful Lens Wear
Adequate corneal oxygen is just one of many
factors that contribute to contact lens success.

Goodlaw (1946) was one of the first individuals to hypothesize that contact lenses act as a barrier to the eyes' anterior oxygen supply. Inadequate oxygen, or hypoxia, can result in the development of a number of contact lens-related complications. This realization has stimulated a fervent effort to develop contact lens materials capable of minimizing the adverse effects induced by corneal hypoxia.
Only recently, with the introduction of silicone hydrogel lenses, has the contact lens industry succeeded in producing materials able to provide enough corneal oxygen to virtually eliminate hypoxia-related complications, while at the same time providing a surface that is hydrophilic enough to permit comfortable wear.
|
|
|
Figure 1. Relationship of EOP to Dk/t across a range of oxygen values. Courtesy of William J. Benjamin, OD, PhD. |
Oxygen Isn't Everything
While the introduction of silicone hydrogels has allowed for the provision of an oxygen-rich environment, it hasn't eliminated complications or guaranteed successful lens wear. Reports have surfaced of microbial keratitis, contact lens-related papillary conjunctivitis (CLPC), superior epithelial arcuate lesions (SEALs) and infiltrative keratitis occurring in patients wearing silicone hydrogel lenses. Some of these conditions, such as SEALs and CLPC, may result from mechanical properties of the lens such as the increased modulus of elasticity, or stiffness, inherent to many silicone hydrogel materials. Even in the absence of ocular complications, patients may still fail to succeed with lens wear secondary to discomfort, dryness, poor vision or handling.
With the recent increased focus on the benefits of delivering increasingly higher amounts of oxygen to the corneal surface, it's important to not overlook the fact that at some point there's a diminishing return of this benefit. In fact, with the trade off of higher oxygen resulting in a higher modulus, non-oxygen related complications might occur more frequently with a higher-modulus lens than they would in a conventional hydrogel. A perfect hydrogel lens would allow for adequate oxygen to minimize hypoxic complications, yet it would retain beneficial conventional hydrogel characteristics such as low modulus to help maximize successful lens wear.
Quantifying Oxygen Delivery
|
TABLE 1 Oxygen Transmissibility Ranking and Equivalent EOP Values* |
||
| RANKING | DK/T RANGE | HUMAN EOP RANGE |
|
Low Dk/t |
<12 | <6% |
| Medium Dk/t | 12-25 | 6-11% |
|
High Dk/t |
26-50 | 11-15% |
|
Super Dk/t |
51-80 | 15-18% |
|
Hyper Dk/t |
>80 | >18% |
| * From Benjamin WJ and Karkkainen TR. Hydrogel hypoxia: where we've been, where we're going. Contact Lens Spectrum. 1996;11(Suppl):s6-11. | ||
Dk Understanding how much oxygen a lens delivers to the cornea, and more importantly how much oxygen the cornea uses, can be confusing because of the various terminology used. Clinicians are most familiar with the manufacturers' listed permeability, or Dk values, but these values can be misleading in a clinical sense because by definition they are a property of the material and independent of the lens thickness. Therefore, when using Dk values you can compare only lenses of identical thickness, which is difficult because of the varying thickness of different designs and powers.
Dk/t A more useful value for clinicians is the Dk/t, or transmissibility, which takes into account the lens thickness. The transmissibility value defines the amount of oxygen that is transmitted through a contact lens in-vitro. Even though this value is more clinically relevant than the Dk value is, it's still problematic. In many cases the only published lens thickness is the center thickness, which allows for information on how much oxygen is delivered only to a small central region of the cornea and ignores the oxygen performance of the lens over its entire diameter.
EOP Both of the above mentioned terms are derived from in-vitro techniques and provide little information on how the cornea actually uses the oxygen provided. To get a better understanding of in-vivo oxygen usage, we can refer to the equivalent oxygen percentage (EOP) value for the lens. EOP values represent an indirect estimate of the oxygen concentration beneath a contact lens.
The first step in determining EOP is measuring the corneal oxygen uptake rate by placing a membrane-covered Clarke-type sensor against the corneal surface and determining the rate at which the cornea consumes oxygen passing through the sensor's membrane. Contact lenses that transmit little oxygen, such as PMMA lenses, would have very rapid oxygen uptake rates, while a cornea not wearing a lens would deplete the oxygen in the sensor at a much slower rate. Researchers can then compare the uptake rates for a particular contact lens to uptake values obtained with known oxygen concentrations to derive the EOP. Thus a lens that gives an EOP value of 10 percent would provide the cornea with approximately half of the available atmospheric oxygen (20.9 percent).
In-vivo EOP values and in-vitro Dk/t values are related; however the relationship isn't linear throughout the entire range of oxygen values. Figure 1 presents the relationship between EOP and Dk/t. Table 1 contains rankings of oxygen transmissibility and their equivalent EOP values, from very low to hyper transmissible. At first glance it may not make sense why the Dk/t vs. EOP graph should asymptote, or flatten out, at the higher ranges of oxygen transmission. After all, if you increase the transmission of oxygen through the lens, wouldn't you expect a greater amount of oxygen to be available underneath the lens?
Upon further consideration, the "fall off" in EOP values with increasing Dk/t does indeed make sense as the EOP values are derived from corneal oxygen consumption, and as the supply of oxygen closes in on meeting corneal demand, the uptake will slow and eventually reach a steady state. The cornea behaves like other biological systems in that there are rate-limiting steps.
Oxygen Flux Another term used in quantifying oxygen delivery, oxygen flux also helps to explain the asymptotic portion of Dk/t vs. EOP graph. Oxygen flux is the volume of oxygen reaching an area of the corneal surface over a given period of time and is represented by Fick's Law, which states:
j=(P1–P0) x Dk/t
where j is the oxygen flux, P1 is the partial pressure of oxygen at the front surface of the lens, P0 is the partial pressure of oxygen at the back surface and Dk/t is the transmissibility. Oxygen flux relies not only on the transmissibility of oxygen through the lens, but also on the pressure at the front surface and back surface of the lens as it rests on the eye. As more oxygen transmits through the lens, there's a resultant rise in P0 and a subsequent decline in the oxygen flux through the lens. The oxygen flux vs. Dk/t graph also becomes asymptotic beyond a certain range of transmissibility in a similar fashion to the EOP vs. Dk/t graph.
|
|
|
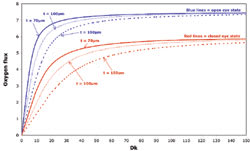
Figure 2. Relationship of oxygen flux and oxygen permeability for a range of lens thicknesses in open and closed eye states. Reprinted with permission from Optician. |
Oxygen: How Much is Enough?
Defining critical oxygen tensions depends upon what physiological indicator you use as your endpoint. Using corneal swelling as the endpoint, Holden and Mertz determined that the minimum transmissibility values required to prevent corneal edema were 24 and 87 Dk/t units for the open and closed eye conditions, respectively. If loss of corneal sensitivity is the physiological indicator, an epithelial partial pressure of oxygen of at least 55 mmHg is likely required from a daily wear contact lens to avoid a significant losses of sensitivity. Table 2 lists critical lens transmissibility values from a number of studies using different physiological endpoints.
Upon inspection of Table 2, it may seem surprising to see such a large range of Dk/t values given as critical values to ensure ocular physiology homeostasis. However this difference is mainly a result of using Dk/t terms to define these critical values. A recent article (Morgan and Brennan, 2004) shows that the large difference in critical Dk values published for the various studies is in reality very small when using oxygen flux values instead of Dk. These different studies then are in general agreement as to the minimum oxygen requirements in terms of flux values.
So how much oxygen is enough? The graph in Figure 2 from Morgan and Brennan shows that for both the open and closed eye state there's a rapid increase in oxygen flux with increasing Dk. However, as Dk continues to increase, a gradual leveling off of oxygen flux occurs with just about all of the conditions becoming asymptotic at a Dk of approximately 30 for the open eye and 80 for closed. These values are in close agreement with Brennan's (2005) conclusion that increases in Dk/t beyond the Holden-Mertz criteria of 24 and 87, for the open and closed eye contact-lens-wearing states respectively, result in minimal oxygen gains. It would be difficult to recommend a magic cut-off value for oxygen transmissibility, especially in lieu of individual responses, but from the above discussion it's evident that once the hypertransmissible area of the curve is reached, increases in Dk/t beyond this value certainly have less of an impact in terms of oxygen utilization.
Non-Oxygen Considerations for Successful Contact Lens Wear
|
TABLE 2 Critical Oxygen Values for Various Physiological Indicators |
|||
| AUTHOR | INDICATOR | DK/T | FLUX |
| Holden (1984) | 4% corneal edema-closed eye with extended wear | 87 | 5.57 |
| Harvitt (1999) | Absence of epithelial anoxia during extended wear | 89 | 5.58 |
| Papas (1998) | Absence of induced limbal hyperemia with extended wear | 125 | 5.73 |
| Giasson (1994) | Absence of epithelial pH change | 300 | 5.94 |
In our rush to cast aside conventional hydrogels and embrace the hypertransmissibility era, it's wise to not forget desirable attributes of conventional hydrogels. After all, if oxygen was the only determinant of successful wear, how could we explain patients who were successful in the PMMA era of lens wear, where no oxygen passed through the lens? PMMA lenses relied on the constant exchange of oxygen rich tears with each blink to provide the cornea with a whopping 3 percent EOP environment! Yet, instances of microbial keratitis in these patients were very low. Oxygen is indeed only one component of successful contact lens wear.
We can determine what lens attributes are needed for successful lens wear by considering why patients discontinue lens wear in the first place. In a survey of contact lens dropouts, the number-one reason cited for discontinuing hydrogel lens wear was discomfort. We can divide comfort into the initial comfort upon lens application and the comfort that is maintained until the end of the day.
Initial lens discomfort may result from a poor fit, lens defect, solution hypersensitivity, lens design or material. You can readily identify these causes of initial discomfort by slit lamp inspection or you can empirically diagnose them by switching to a different lens.
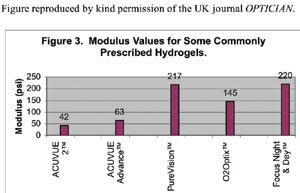
Mechanical characteristics of a lens may also affect initial comfort levels as evidenced by anecdotal reports of lens awareness when subjects initially switch from conventional hydrogels into silicone hydrogels having a higher modulus value. The stiffness of these materials may be responsible for initial lens awareness, which usually subsides with continued wear. Figure 3 shows the modulus values of some commonly prescribed hydrogel lenses.
It thus makes sense that a silicone hydrogel lens may provide better initial comfort if has a low modulus similar to that found in conventional lenses. According to Figure 3, the only silicone hydrogel lens currently on the market that has a modulus in the range of conventional hydrogels is Acuvue Advance with Hydraclear. Reduced modulus also offers another benefit of potentially avoiding adverse mechanical lens effects such as SEALs and CLPC as discussed earlier.
Numerous factors such as the lens fit, deposit build-up or reports of end-of-day dryness may affect end-of-day comfort. Lenses that show no or inadequate movement will tend to become more uncomfortable throughout the day. A lens that moves freely over the cornea and quickly returns to its original resting position is optimal in terms of movement. Remember that lens movement depends on the peripheral design of the lens and to a lesser extent on base curve; therefore, switching to a flatter base curve may not ensure more movement, and you may have to try a different lens.
Deposits may also cause contact lens discomfort. With the adoption of frequent replacement contact lenses, the problem of deposit-related discomfort has become less of an issue for both patients and doctors.
End-of-day discomfort and dryness is a common complaint of many lens wearers, especially those who are diagnosed with dry eye. Some lenses such as CooperVision's Proclear Compatibles are targeted at the specific problem of contact lens-related dryness. The lens contains phosphorylcholine (PC), which is thought to bind water molecules and lead to greater water retention and less on-eye dehydration. Acuvue Advance is another lens that binds water to the hydrogel. Hydraclear is a long-chain, high-molecular-weight, hydrophilic molecule (PVP) that acts as a humectant (moisture loving agent) and lubricant, allowing for incorporation of silicone into the hydrogel, increasing oxygen transmissibility while still providing a wettable lens surface and moisture retention without negatively affecting lens stiffness.
|
|
|
Figure 3. Modulus values for some commonly prescribed hydrogels. |
Hyperemia, vision, handling and economics can also play a role in contact lens dropout. Pritchard, et al (1999) listed red eyes in addition to discomfort and dryness as primary reasons for lens discontinuation. Although ocular hyperemia may result from many etiologies, hypoxia has been implicated in the development of limbal hyperemia. A recent study (Maldonado-Codina et al, 2004) showed that Acuvue Advance did not increase limbal hyperemia from the baseline non-lens wearing eye in a group of neophyte contact lens patients.
The Right Combination
Although increasing oxygen transmissibility to meet critical physiological needs is vital, once these needs have been met additional increases will have a diminishing benefit. Your first duty is to provide the highest quality of care to your patients and make recommendations based on what you feel is in their best interest. I believe you can best accomplish this by taking a holistic approach and weighing the risks and benefits of each treatment that you offer to your patients.
Although often trivialized, contact lenses are medical devices and can be associated with sight-threatening complications. Therefore, you must evaluate the risks and benefits of lenses you recommend for your patients, with the ultimate goal to provide clear, comfortable vision throughout the day while minimizing the risk of complications. Keeping this in mind will ensure patient satisfaction and a successful contact lens practice.
To obtain references for this article, please visit http://www.clspectrum.com/references.asp and click on document #126.
Dr. Artis is the director of Professional Affairs for Vistakon, division of Johnson & Johnson Vision Care, Inc.