MPS COMPARISON
A
Comparison of Two Lens Care Formulations
This
study shows that a newer solution may improve comfort, wear time and visual
acuity for some patients.
By Robert P. Wooldridge, OD, Howard I. Schenker,
MD, Lee E. Rigel, OD, Kenji Hamada, OD, and S. Barry Eiden, OD
|
|
|
Figure 1. Group I and Group IV lens wearers using Opti-Free Replenish had consistently fewer eyes with grade 2 or higher corneal staining. |
The next generation of lens care solutions has the goal of enhancing patient comfort and safety. Manufacturers are designing new solutions that condition lenses and keep them clean and comfortable longer.
Latest Arrival
The newest solution to incorporate an integrated conditioning system is Alcon Laboratories' Opti-Free Replenish Multi-Purpose Disinfecting Solution (MPDS), which incorporates the same patented disinfecting system — Polyquad 0.001% and Aldox 0.0005% — as the company's Opti-Free Express formula. Replenish also continues to use citrate as a cleaning agent.
TearGlyde, the new proprietary conditioning system in Opti-Free Replenish, is composed of the surface active agents Tetronic 1304 and C9-ED3A (nonanoyl ethylenediaminetriacetic acid). According to Alcon, this new system reconditions the lens surface during each overnight soak, positively affecting wettability (the attraction between the lens surface and the tears). Increasing the affinity of tears to lens surfaces should improve comfort, increase visual performance and increase overall patient satisfaction. C9-ED3A also helps to prevent calcium build-up on lenses and to enhance cleaning, replacing EDTA in the formulation. Opti-Free Replenish also incorporates propylene glycol, a demulcent, to enhance wetting, lubrication and comfort. Propylene glycol helps bind water inside the lens and is commonly used in artificial tear products.
To be useful, new products must offer improvements upon currently available products. The purpose of this 90-day clinical study was to evaluate the safety and efficacy of Opti-Free Replenish MPDS compared to ReNu MultiPlus MPS No Rub Formula.
|
|
|
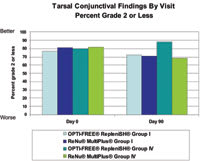
Figure 2. Group IV wearers showed better tarsal conjunctival scores by day 90 with Opti-Free Replenish. |
Statistical Methods
We used analysis of variance to test for differences between treatment regimens in mean residual lens lysozyme deposits on Group IV lenses worn by subjects. We used a repeated measures analysis of variance to compare regimen differences for continuous variables (Likert questionnaire items and average/uncomfortable lens wearing time). To assess differences between regimens by lens group for categorical variables (Rudko and lens replacement incidence), we performed a Chi-Square test of independence. To assess differences in distribution of the corrected visual acuity and the tarsal conjunctiva slit lamp findings, we used a Cochran-Mantel-Haenzel rank score test, and we performed a two-sample t-test on residual lens lysozyme levels from Group I lenses.
Study Methods
Twelve optometry and ophthalmology practices evaluated Opti-Free Replenish MPDS in a three-month, double-masked, IRB approved clinical study. Before participating in the study, investigators obtained consent and then fit experienced soft contact lens wearers who had normal eyes with either Optima 38 lenses (Bausch & Lomb — Group I, low-water, non-ionic material) or Hydrasoft EW lenses (CooperVision — Group IV, high-water, ionic material). Patients who could not successfully adapt to the new lenses after seven days were not enrolled in the study. Investigators enrolled all other patients (day 0) and fit them with a new pair of Optima 38 or Hydrasoft EW lenses to be worn without replacement for the next three months. Study participants were representative of the general lens wearing population in age and gender.
Investigational site staff members responsible for dispensing the solutions and instructions randomized 125 study participants to Opti-Free Replenish MPDS and 127 participants to the control, ReNu MultiPlus MPS. To mask their identity, the staff members packaged the solutions in plain white bottles with non-branded, "investigational use only" labels. The evaluating doctors remained unaware throughout the study of which product each patient had received. Investigators provided rewetting drops, either Opti-Free Express Rewetting Drops or ReNu Rewetting Drops, also in masked bottles, for use at the patients' option. At day 0, patients completed a self-administered Likert-style questionnaire that used a five-point scale (strongly disagree to strongly agree) to describe their baseline comfort. Investigators performed baseline slit lamp and visual acuity measurements.
|
|
|
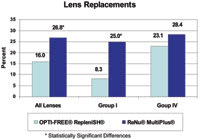
Figure 3. Significantly fewer lenses required replacement for patients using Opti-Free Replenish. |
At follow-up visits on days 14, 30, 60 and 90, patients completed a questionnaire using a five-point Likert-style scale to describe comfort over the respective previous three days. Investigators scheduled follow-up visits at approximately the same time of day and instructed patients to wear their lenses from two to four hours before the examination. This stipulation decreases variability in signs and symptoms that occur over the course of the lens wearing day and maximizes the ability to detect corneal staining with some lens/solution combinations.
The investigating doctors asked the patients about wear time and compliance with the test regimen; evaluated corrected visual acuity; conducted a slit lamp examination that included corneal staining, edema, neovascularization, injection and infiltrates; and classified the tarsal conjunctiva using the Lofstrom-Kruse scale. They graded corneal staining using a 0-4 scale where 0 = none, 1= trace, 2 = mild, 3 = moderate and 4 = severe. Investigators also evaluated lens cleanliness (modified Rudko) at each visit.
When patients exited the study, investigators placed their study lenses in new screw-top cases and shipped them overnight to the sponsor for high performance liquid chromatography (HPLC) analysis to determine the amount of lysozyme in the lenses. We masked the laboratory workers testing the lenses as to which solutions had been used with the lenses.
Results
|
|
|
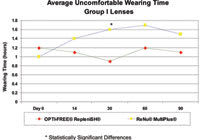
Figure 4. Differences in uncomfortable wear time favoring Opti-Free Replenish became significant at day 30 and showed numerical differences at day 60 and day 90. |
Safety Safety results for Opti-Free Replenish MPDS were consistent with results historically seen in studies with Opti-Free Express. Both Opti-Free Replenish and ReNu MultiPlus were safe and well-tolerated. Two patients (1.6 percent) using Opti-Free Replenish and eight (6.3 percent) using ReNu MultiPlus experienced ocular complications related to the solutions, primarily discomfort. Five patients using ReNu MultiPlus were discontinued from the study for reasons relating to the test solutions (ocular discomfort, dry eye, hyperemia, conjunctival papillae and corneal staining). None of the patients using Opti-Free Replenish were discontinued for solution-related reasons.
Injection and corneal staining were the only slit lamp findings reported as clinically significant complications during the study and were observed in two (1.6 percent) patients using Opti-Free Replenish and five (3.9 percent) patients using ReNu MultiPlus. In addition, Group I and Group IV lens wearers using Opti-Free Replenish had consistently fewer eyes with grade 2 or higher corneal staining compared to ReNu MultiPlus users at all follow up visits (Figure 1). Group IV lens wearers showed better tarsal conjunctival scores by day 90 with Opti-Free Replenish than with ReNu MultiPlus (Figure 2). At day 90, 88.1 percent of Group IV lens wearers using Opti-Free Replenish had tarsal grades of 2 or lower compared with 68.9 percent of those using ReNu MultiPlus. Investigators observed no other clinically relevant differences in slit lamp findings between regimens during the study.
Lens Replacements We selected Hydrasoft EW and Optima 38 contact lenses for this study because they can more likely withstand the FDA's requirement of daily handling for 90 days without replacement. In this regard, 84 percent of the lens wearers using Opti-Free Replenish and 73 percent of the lens wearers using ReNu MultiPlus completed the study with both original contact lenses intact. As Figure 3 shows, significantly fewer lenses required replacement for patients using Opti-Free Replenish MPDS (16 percent) than for patients using ReNu MultiPlus (26.8 percent), p=0.037. Significantly fewer Optima 38 lenses (Group I) required replacement when subjects used Opti-Free Replenish (8.3 percent) than when patients used ReNu MultiPlus (25 percent), p=0.01. There were no significant differences between solutions for Group IV contact lens replacements.
|
|
|
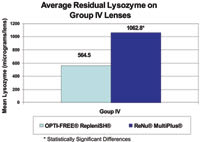
Figure 5. Group IV lenses cleaned with Opti-Free Replenish had significantly less lysozyme. |
Wearing Time and Comfort At each visit, investigators asked study patients on average how many hours they had worn their contact lenses each day and how many hours the lenses had been uncomfortable. The mean reported wearing time for each lens type and solution group was maintained at 13 to 14 hours throughout the study. The average uncomfortable wearing time at the baseline examination ranged from 1 to 1.4 hours. Opti-Free Replenish was more successful than was ReNu MultiPlus in keeping average uncomfortable lens wearing time stable with baseline for Group I lens wearers. Differences in uncomfortable wear time favoring Opti-Free Replenish became significant at day 30 (p= 0.03) and showed numerical differences at day 60 and day 90 (Figure 4). We found no significant difference in average uncomfortable wear time with Group IV lenses.
A review of the Likert questionnaires that patients used to describe their ocular comfort at each visit showed that comfort and satisfaction remained at high levels throughout the study with both solutions. We found the most consistent difference with the statement "This solution is gentle on my eyes," for which patients wearing Group IV lenses rated Opti-Free Replenish significantly higher than ReNu MultiPlus overall (p<0.01) and at days 60 (p<0.03) and 90 (p<0.01).
Responses to the statement "When I use this solution my lenses feel moist" were significantly more favorable overall with the Opti-Free Replenish regimen compared to the ReNu MultiPlus regimen (p=0.03) and at day 60 (p<0.01) among Group IV lens wearers. When we combined Group I and Group IV contact lenses across all visits, mean responses by patients using Opti-Free Replenish were significantly more favorable for the statements "When I use this solution my lenses feel moist" (p=0.04) and "This solution is gentle on my eyes" (p=0.04). Responses to all other Likert statements showed no significant difference between solutions.
Visual Acuity and Lens Cleanliness Opti-Free Replenish and ReNu MultiPlus successfully maintained corrected visual acuity with baseline values throughout the 90-day study. We found no differences between Opti-Free Replenish and ReNu MultiPlus with Group I lenses. With Group IV lenses, we observed a greater prevalence of worsening in visual acuity from baseline for ReNu MultiPlus at day 14 (p=0.02), with numerical differences noted at days 30, 60 and 90.
Like its predecessor Opti-Free Express, Opti-Free Replenish was highly effective at keeping lenses clean throughout the 90-day study as measured by both objective HPLC measurement of protein in the lenses at day 90 and subjective Rudko evaluations conducted by the investigators. HPLC analysis measures the amount of lysozyme, the primary protein in tears, in a contact lens. Opti-Free Replenish proved to be an effective cleaning solution with significantly less residual lysozyme compared to ReNu MultiPlus (Figure 5). After 90 days, we found 47 percent less residual lysozyme in the Group IV lenses treated with Opti-Free Replenish (mean = 564.5μg) compared to lenses treated with ReNu MultiPlus (mean = 1062.8μg, p<0.0001). We saw no significant difference in residual lysozyme with Group I lenses, which absorb much less protein.
|
|
|
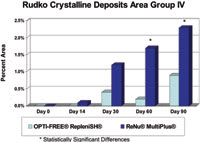
Figure 6. The mean percent area of crystalline deposits on Group IV lenses was significantly less for the Opti-Free Replenish MPDS regimen at day 60 and day 90. |
Visual examination of the contact lenses, conducted at each study visit, paralleled the HPLC results. Investigators classified lens deposits according to the modified Rudko scale. They found no significant differences with Group I lenses. The percentage of patients with Group IV lenses that investigators classified as microscopically clean (Rudko Type 1) was significantly higher (p=0.02) for the Opti-Free Replenish group at day 90 compared to the ReNu MultiPlus group. Investigators noted no significant differences in lens area with film deposits among the treatment regimens at any visit for either lens type. The mean percent area of crystalline deposits (Rudko Type 2-4) on Group IV lenses was significantly less for the Opti-Free Replenish MPDS regimen at day 60 (p=0.01) and day 90 (p=0.03) (Figure 6).
Discussion
Results indicate that Opti-Free Replenish MPDS offers an advance over the compared multipurpose solution. We believe the addition of TearGlyde enhances comfortable wear and reconditions the lens surface to retain moisture. Use of Opti-Free Replenish was associated with excellent comfort, lens cleanliness, visual acuity and patient satisfaction. Corneal and tarsal findings with Opti-Free Replenish were consistent with or better than baseline and the control solution, ReNu MultiPlus MPS.
We believe Opti-Free Replenish MPDS offers clear advantages over ReNu MultiPlus, especially for the 70 percent of traditional soft contact lens wearers who wear Group IV lenses. Opti-Free Replenish maintained visual acuity more consistent with that observed with new lenses. Microscopic deposits were fewer, and at the end of the study we recovered 47 percent less protein from the contact lenses cared for with Opti-Free Replenish. Study patients consistently rated Opti-Free Replenish as "gentle on my eyes." Group IV lens wearers also trended toward more agreement with the statement "with this solution my lenses feel moist" with Opti-Free Replenish.
Patients who suffer dryness and discomfort are more likely to become dissatisfied and to drop out of contact lens wear. TearGlyde in Opti-Free Replenish is designed to recondition the lens surface during every overnight soak so that patients may achieve a high level of lens surface moisture for all-day comfortable lens wear. Recent studies have shown that Opti-Free Replenish maintains lens surface wettability for 14 hours after 30 days of lens wear.
As the lenses aged over the course of the study, total wearing time did not change — but uncomfortable wearing time remained more consistent with baseline with Opti-Free Replenish. This was especially evident with Group I lens wearers at day 30, where average uncomfortable lens wearing time was statistically significantly lower for Opti-Free Replenish than for ReNu MultiPlus.
Potter et al (2005) have shown that Opti-Free Replenish MPDS with TearGlyde increases comfort for wearers of the most common soft contact lens material who experience discomfort. Our study showed that the solution provides substantial benefits with asymptomatic lens wearers compared to ReNu MultiPlus. The solution is also designed to provide safe and effective cleaning, storage and enhanced disinfection, yet is gentle enough for sensitive eyes.
Alcon Laboratories sponsored this study. All authors served as principal investigators for this study.
To obtain references for this article, please visit http://www.clspectrum.com/references.asp and click on document #132.
Dr. Wooldridge is the Director of The Eye Foundation of Utah, an optometric co-management center in Salt Lake City, Utah, and is an adjunct clinical professor at several schools and colleges of optometry.
Dr. Schenker is an associate clinical professor at the University of Rochester. He is a fellowship trained glaucoma specialist, and his areas of research include glaucoma, contact lenses, dry eye and allergy.
Dr. Rigel is located in E. Lansing, Mich., where he practices in a three doctor office specializing in contact lenses. He also participates in clinical investigational studies for several contact lens manufacturers and pharmaceutical laboratories.
Dr. Hamada is co-founder of a group practice located in Grants Pass & Cave Junction, Oregon. He has been a clinical investigator and consultant and served on numerous contact lens and pharmaceutical panels.
Dr. Eiden is president of North Suburban Vision Consultants, Ltd., a private group practice specializing in primary eye care, contact lens management, treatment of eye diseases and refractive surgery. He is also an assistant clinical professor at the University of Illinois at Chicago Medical Center in the Department of Ophthalmology, Cornea and Contact Lens Service.