CONTINUING EDUCATION
Ocular and Refractive Considerations for the Aging Eye
A review of physical and refractive changes that occur in the eye over time and available corrective options.
BY KATHRYN RICHDALE, OD, MS, FAAO
The incidence and prevalence of ocular diseases such as glaucoma and macular degeneration increase with age and can cause devastating effects on vision. Even normal age-related changes of the ocular structures can affect visual function.
This article discusses typical aging changes in the ocular system and how they impact the treatment and management of your patients, especially those who desire contact lens wear.
Vision and Refractive Error in Adults
For most people, significant changes in refractive error subside in adolescence or early adulthood. What is often overlooked is that a shift toward hyperopia occurs later in life. From about the fourth through the sixth decades of life, spherical refractive error changes by about +0.05DS per year, or +0.50DS per decade. It's not until the 70s or 80s that a "myopic shift" of up to about –0.70DS occurs (Figure 1). Refractive astigmatism also changes during adulthood at the rate of about 0.25DC per decade. The orientation of refractive astigmatism generally shifts from "with-the-rule" to "against-the-rule" (Figure 2). Anisometropia of more than 1.00DS also increases with age.
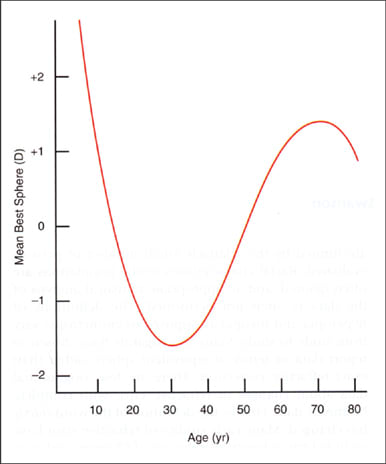
Figure 1. The average change in mean spherical equivalent refractive error with age. (From H. Saunders Opthalmic Phys Opt 6:39-46.)
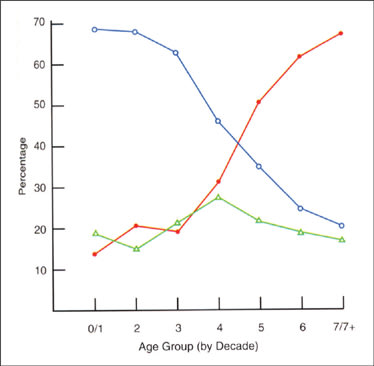
Figure 2. The change in refractive astigmatism with age. "With-the-rule" astigmatism is plotted in blue, "against-the-rule" in red and oblique in green. (From H. Saunders Opthalmic Phys Opt 8:37-42.)
In addition to the changes in lower-order aberrations of nearsightedness, farsightedness and astigmatism, changes in higher-order aberrations (HOAs) also occur with age. In general, spherical aberration becomes more positive and total HOAs increase with age. Changes in HOAs can cause symptoms of glare, halos and ghosting.
The Eye Diseases Prevalence Research Group recently estimated that for the United States population of adults age 40 years and older, the prevalence of hyperopia greater than +3.00D was about 10 percent and myopia greater than –1.00D was about 25 percent. In addition, most symptoms of presbyopia begin in the mid-40s. The loss of accommodative function is nearly complete by 60 years of age. These slowly progressing but significant changes in refractive error, aberrations and accommodative function may impact the long-term effectiveness of treatments such as refractive and cataract surgery.
Best-corrected visual acuity is relatively stable until about age 50, and then declines as a function of age. Although it's difficult to separate normal age-related changes from those of ocular disease, the results of the Beaver Dam Eye Study demonstrate that about one in five patients over age 65 has impaired vision of 20/60 or less in the better eye. Visual impairment and legal blindness are even greater problems in the nursing-home population. There is a marked decrease in contrast sensitivity at both medium and high spatial frequencies with age. The average 60-year-old patient receives only onethird of the light to the retina compared to a 20-year-old. Both optical and neurological changes are responsible for the decline in visual acuity and function. Normal age-related changes in the ocular system can affect your patients' performance and satisfaction with their vision correction. Consider the benefits and drawbacks of each modality when recommending spectacles, contact lenses or surgery for your adult patients.
Changes in the Ocular System with Age
Anterior Segment A marked decline in elasticity of the eyelids occurs with age. This may cause ectropion, entropion, dermatochalasis and ptosis. With age, both the upper and lower lid move downward and the palpebral apertures decrease in size. A loss of tonus in the lids may cause patients to have difficulty with GP contact lens application and removal. Problems with lid apposition or a change in lid position can contraindicate the fitting of certain GP contact lens designs. Consider these factors before recommending corrective options for patients.
There is no significant change in corneal diameter, central corneal thickness, or anterior and posterior corneal curvature with age. If you note a change in corneal thickness or curvature in a contact lens wearer, you should reassess the fit and oxygen permeability of the contact lens material.
Corneal sensitivity declines with age. While this may help with adaptation to contact lenses, especially GPs, it could also increase the risk of complications as patients may not be aware of a problem until an abrasion or ulcer becomes severe. Educate older patients about the decrease in corneal sensation and instruct them on the importance of seeking care if they have any signs of redness or discharge from the eye.
Older adults have an increased prevalence and severity of pterygium and pinguecula, which may alter astigmatic refractive error and interfere with contact lens wear. A significant increase in the signs and symptoms of dry eye occurs in older adults, especially in women. With age, lacrimal glands shrink and there is a decline in the goblet cells of the conjunctiva. This causes a reduction in overall tear secretion.
The biochemical composition of the tear film also changes with age. Systemic disease increases with age, and common medications for hypertension, allergy, anxiety, depression and hormone replacement therapy are known to compromise the tear film. Meibomian gland dysfunction and blepharitis are also more common in older adults. A poor tear film not only affects the patient's visual quality and stability of vision; symptoms of dryness are among the primary reasons that patients discontinue contact lens wear. A tear breakup time less than 10 seconds, corneal or conjunctival staining with fluorescein or lissamine green, or reduced tear volume with Schirmer or phenol red thread test signify an underlying dry eye that should be treated before prescribing contact lenses. Options range from recommending an over-the-counter artificial tear or an increased intake of water or omega fatty acids to inserting punctal plugs or prescribing anti-inflammatory medications.
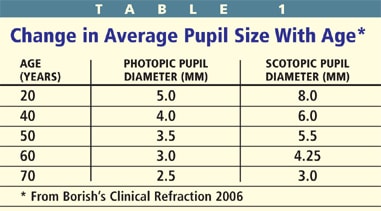
With age, the pupils decrease in size and become less reactive. There are significant variations in pupil diameter among individuals, but "senile miosis" accounts for a decrease of about 2.5mm and 5mm in the light and dark, respectively, from 20 to 70 years of age (Table 1). The change in pupil size has been attributed to either muscle atrophy or a change in sympathetic or parasympathetic innervations. While the reduction in pupil size may be beneficial for increased depth of focus, it may also compromise vision in low lighting conditions. Many of the multifocal contact lens and intraocular lens implants are pupil size dependent, thus you should take pupil measurements in normal and dim illumination prior to recommending a presbyopic correction.
Presbyopia The decline in accommodation with age has been studied for more than a century. Duane's study of more than 4,000 patients in the early 1900s demonstrated the almost perfectly linear decline in accommodation with age (Figure 3). Presbyopia is the only refractive error with a 100-percent incidence rate, given a life-span of at least 50 years.
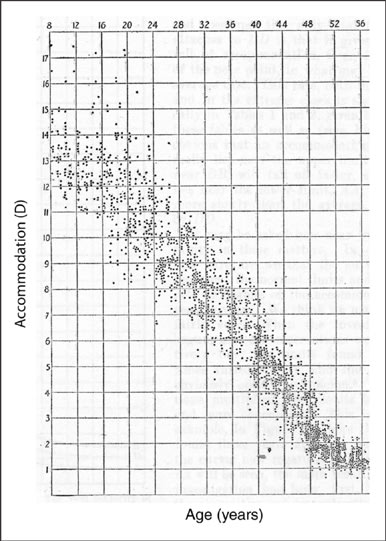
Figure 3. Duane's study of subjective accommodation and age demonstrates the linearity in accommodative decline (From the Normal Values of the Accommodation at all ages; Alexander Duane, Journal of the AMA, 1912).
Despite being the most common ocular refractive condition, there is still a limited understanding of the complete process of accommodative loss. In Helmholtz's well-accepted theory of accommodation, the ciliary muscle contracts, causes a reduction in zonular tension and allows the crystalline lens to become more curved and higher powered. Helmholtz's idea that presbyopia is due to age-related sclerosis of the lens is not as well accepted and has been challenged by research that suggests that changes in the ciliary body, zonules and/or arrangement of the structures of the accommodative system result in accommodative decline. Thanks to improvements in technology we now know that all of the accommodative structures change either their structure or function with age (Figure 4).
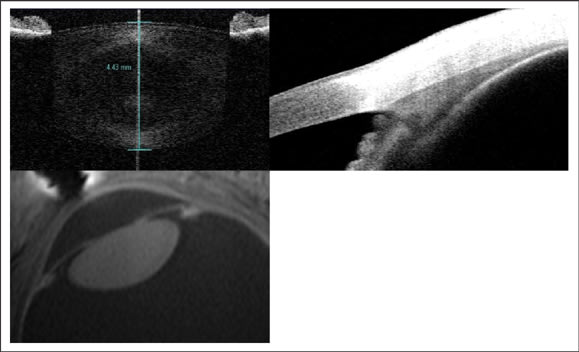
Figure 4. The crystalline lens, ciliary body and geometry of the accommodative structures change with age. In vivo imaging allows researchers to study changes in the human eye. The top images are of the lens (left) and ciliary body (right) obtained with the Visante anterior segment optical coherence tomographer. The lower image is of the arrangement of the accommodative structures obtained with ultra-high field MRI.
With normal aging there is an increase in brunescence of the lens and a corresponding increase in light scatter, especially at shorter wavelengths. The crystalline lens continues to create new cells and to grow throughout life, increasing in thickness by about 18-to-30 μm/year. The increase in lens size alone has been shown to be related to accommodative function. Lens growth occurs almost entirely in the anterior and posterior cortex, with little change to the lens nucleus. Magnetic resonance imaging studies show that there is little, if any, change to the lens equatorial diameter in adulthood. The equivalent refractive index of the lens decreases with age, which partially offsets the power increase that would occur with a thicker and more curved lens; this is often referred to as the "Lens Paradox."
With age, the crystalline lens becomes stiffer and is less easily molded. While the increase in lens stiffness likely accounts for some of the accommodative decline in humans, the loss of lens elasticity and decline of accommodation reported by many studies is exponential, contrary to the linear decline in accommodative response. Furthermore, the major increases in lens stiffness may occur beyond the age of 50 years, long after the majority of the accommodative function is lost.
The pupil and iris muscle decrease in size and responsiveness with age. Thus, it seems reasonable that the ciliary body, a related intraocular muscle, would also change with age. Histological reports show that with age, both the longitudinal and reticular portions of the ciliary muscle decrease in size, leading to an overall decrease in length by as much as 2mm. The apical edge of the ciliary muscle moves anterior and inward, closer to the lens. A recent study found that, while the longitudinal portion of the muscle may continue to contract throughout life, the circular and radial portions exhibit decreased contractility with age. The age-related changes in the ciliary body create an arrangement of the accommodative structures comparable to that of the young, accommodated eye. The ciliary body also shows an increase in connective tissue beginning in the third decade of life, the accumulation of lipofuscin in the fifth decade, and atrophy of the muscle after 60 years of age. It seems that the ciliary muscle continues to contract throughout life, but the amount and direction of useable force is uncertain.
The zonules transfer the force of the ciliary muscle to the lens and are essential to the modification in lens shape. Previous studies have yielded mixed results regarding changes in the zonules with age. Some have stated that zonule elasticity does not change between the ages of 15 and 45 years, while others have found that the zonules enter more anterior on the lens and are thinner, fewer and more "easily disrupted" with age.
Visual Correction in Presbyopic Patients
Contact Lens Options The options for presbyopic contact lens correction have expanded significantly in the past decade. There are now GP, traditional hydrogel and silicone hydrogel contact lens options. Current contact lens wearers often want to continue with contact lenses into their 40s and beyond. Frequent reports have indicated that the current generation of presbyopes is averse to any indication that they are aging and desires to retain a healthy and active lifestyle. While you should inform patients that there is no "perfect" treatment or cure for presbyopia, presbyopic contact lens correction should meet "most of their visual needs, most of the time," but there will be times when spectacles are preferable. There are few contraindications to presbyopic contact lens wear, but success is greatest in patients who are current lens wearers, who are motivated to wear contact lenses, and who do not suffer from dry eye or other ocular diseases that compromise vision or contact lens fit.
It is critical to start any presbyopic contact lens fitting with a careful refraction. While ±0.25D doesn't make much of a difference for a non-presbyopic patient, it can have a significant impact on vision and satisfaction with multifocal lenses. Most patients are becoming more hyperopic during this stage, and over-minusing the patient may lead to the use of a higher-than-necessary add power.
After determining the refraction, apply the correction for vertex distance and select the spherical equivalent for the initial distance contact lens power. Do not use a spherical design if the cylinder power is more than 1.00D. Uncorrected astigmatism may be acceptable in younger patients, but with the changes in HOAs the addition of uncorrected cylinder can create too much visual compromise in a presbyope. Determine the add requirements with binocular cross cylinder testing, NRA/PRA, age, etc. and check eye dominance for every patient. To check for dominance, make sure the patient is wearing the full distance correction and use a sighting method or the plus lens test.
It is even more important with presbyopic contact lens modalities to allow the lenses to settle for at least 10-to-20 minutes before checking fit and vision. If the fit is acceptable, check vision binocularly at distance and near. Perform all vision testing with normal room illumination. Over-refract in free space with hand-held trial lenses. Putting the patient behind a phoropter creates an artificial near accommodative response and alters pupil size. When assessing near vision, use a reading card with sentences in different fonts and sizes, not individual letters. Inform patients that there is a normal adaptation period and that experiencing the vision in their specific work and leisure environments is critical to finding the best prescription. The fitting process is improved when patients take an active role and feel that determining the best contact lens prescription for their visual needs is a joint effort.
Monovision contact lens correction is an option for most patients. The benefits of monovision include ease of fitting, ability to utilize any type of contact lens material with a full range of powers, and minimum lens cost. Monovision is not pupil size dependent and, thus, does not suffer problems of compromised vision in dim lighting or low contrast conditions. The major disadvantage of monovision is the loss of stereoacuity, especially at higher add powers. Also, some patients have difficulty suppressing one eye and are unable to adapt to monovision correction. Before fitting monovision, inform patients of potential problems with driving and depth perception. Over-spectacles may alleviate this problem, assuming they don't induce symptomatic anisometropia.
Studies with both soft and GP lens materials have demonstrated that when patients are given an opportunity to try both monovision and multifocal lens modalities, the majority of patients prefer multifocals. With the availability of so many multifocal options in both GP and soft contact lens materials, monovision fitting is best limited to early presbyopes.
There are now many bifocal and multifocal soft contact lens options in both traditional hydrogel and silicone hydrogel materials with replacement options from yearly to daily disposable. Two silicone hydrogel multifocal lenses are now available for continuous or extended wear. Soft bifocals and multifocals are great options for patients who require clear binocular vision at far, intermediate and near distances; however, you should counsel patients on the possible compromise in vision, especially in low lighting or low contrast conditions. You can demonstrate to patients how changes in lighting and working distance can improve visual function.
Most presbyopic soft contact lenses incorporate aspheric and/or spherical annular portions of the lens and can have either a center-distance or center-near design. Many patients are most successful with a "modified monovision" fit. For example, a patient may wear a higher add power or center-near lens on his non-dominant eye. Alternatively, you can "over-minus" a patient in the dominant eye to effectively reduce some of the add power or "over-plus" the non-dominant eye to improve near vision. Early presbyopes may do well with two center-distance contact lenses. If patients have astigmatism greater than about 1.00DC, consider toric monovision, a toric soft multifocal or GP lenses when refractive astigmatism is roughly equal to corneal curvature.
GP bifocal and multifocal lenses have been a mainstay in presbyopic contact lens correction and remain an excellent option for many patients. Both aspheric and translating lens designs are available and, especially with translating designs, offer vision comparable to that of spectacle lenses. For a translating design to work, a patient must have a lower lid that retains some tonus, is well-apposed to the globe, and positioned at or within 1mm of the limbus. Inform patients that they need to drop their eyes, not their head, for near viewing. For mature presbyopes, some translating designs are also available with an aspheric segment for intermediate vision needs.
Aspheric GP multifocals are more commonly prescribed than translating designs, but do suffer from some of the same compromises in vision as soft aspheric multifocals. Early presbyopes and those who do a lot of computer or intermediate work are well-suited for an aspheric design. Absolute presbyopes may not be satisfied with the compromise in distance or near vision with aspheric designs.
Most aspheric GP multifocal contact lenses have a back-surface design and, thus, are center-distance; however, advances in manufacturing have introduced new designs with both front-surface aspheric and concentric ring designs. Some aspheric multifocal GP designs allow the addition of a spherical zone of plus power in the periphery to improve near vision without sacrificing distance acuity. Others allow you to modify the central optic zone diameter to improve distance or near vision based on the patient's pupil size.
The primary fitting consideration with aspheric GPs is an upper eyelid geometry that allows a lid-attached fit. The lens must center well and show limited movement with the blink. The same modified monovision fitting approach can be applied to GP designs. It is important to note that aspheric designs are contraindicated in patients with pupils larger than about 5mm in normal room illumination, as patients may have considerable problems with glare and ghosting. In this case a translating design would be indicated.
Due to the aforementioned changes in pupil size, aberrations and astigmatism, it is important to remember that while a patient may easily read the 20/20 line in the office, he or she may have difficulty with low lighting or low contrast conditions. Changes in lens design, for example from an aspheric to a translating design, can be beneficial. Likewise, if a patient complains of fluctuating vision, be sure to assess the tear quality and quantity and work to maintain a high quality optical refracting surface.
Surgical Options
There is a growing interest in surgical corrective options for presbyopia including monovision, multifocal and accommodating intraocular lens (IOL) implants. Many of the current options for IOLs parallel contact lens corrections. Patients who have been successful with monovision contact lenses may prefer to use single-optic IOLs with one eye corrected for distance and one for near. There are currently two multifocal IOL designs approved for use in the United States. Multifocal IOLs are also pupil dependent and carry some of the same compromises in low contrast and low lighting conditions as contact lenses do. You may suggest combining a singleoptic IOL with a multifocal, or utilizing a different multifocal IOL in each eye if one design has its strengths at intermediate distances and the other at near.
There is one "accommodating IOL" available in the United States. The accommodating IOL consists of two single-optic lenses and a hinged plate haptic. It is implanted within the capsular bag and is dependent on the fact that the ciliary body continues to contract with accommodative effort throughout life. The proposed mechanism of action is that a contraction in the ciliary body creates an increase in vitreous pressure which vaults the lens forward. Due to optics, an average eye may only ever be able to achieve about a +1.00D addition with a single-optic lens design. Theoretically, a dual-optic lens design could allow a higher accommodative response, but there are limitations to the size and movement of this type of IOL in the human eye. Because "accommodating" IOLs are usually made with spherical optics, there should be no compromise in contrast sensitivity or problems with glare or haloes with these designs. Correcting astigmatism is not as straightforward with surgical procedures as with contact lenses. IOL options for patients with significant amounts of astigmatism remain limited. Those patients with smaller amounts of astigmatism may benefit from corneal cylinder correction.
The future of surgical presbyopic corrections is reliant on understanding all of the changes of the accommodative structures with age. The direction and force of the ciliary muscle contraction, transition of force through the zonules, and overall size and shape of the crystalline lens are all significant parameters in the accommodative response. Current research in presbyopic correction includes both single- and dual-optic accommodating IOLs; pliable IOL materials that change curvature, replicating the natural crystalline lens; and the use of laser technology to decrease the stiffness of the mature lens.
Conclusion
The "baby-boom" generation, encompassing more than 76 million Americans, is now presbyopic. According to the U.S. Census Bureau, by the year 2025 approximately half of the United States population will be over 40 years of age. Currently, the cost of providing care to treat the refractive error of patients over 40 is higher than that for treating macular degeneration, diabetic retinopathy and glaucoma combined. Using the National Eye Institute Refractive Error Quality of Life instrument, McDonnell and co-workers showed that presbyopes scored worse than non-presbyopes on seven of the 13 scales, including expectations of future vision, diurnal fluctuations, ocular symptoms, dependence on correction, and overall vision satisfaction.
There is an enormous opportunity for improvements in the treatment options for presbyopia. As healthcare providers, it is imperative that we understand the changes in the ocular system with age, discuss the treatment options available to our patients and guide them in choosing the best vision correction(s) for their needs. CLS
For references, please visit www.clspectrum.com/references.asp and click on document #159.
Dr. Richdale is a senior research associate and clinical attending in the Contact Lens Service at The Ohio State University College of Optometry. Her research is primarily focused on accommodation and presbyopia.



