OCULAR SURFACE DISEASE
Ocular Surface Disease Update 2010
A look at new classifications as well as diagnosis and treatment options for this prevalent disease.
By Blair B. Lonsberry, MS, OD, MEd, FAAO

| Dr. Lonsberry is clinic director and professor at Pacific niversity College of Optometry, Portland, Ore. He is a consultant or advisor to Inspire Pharmaceuticals and Alcon Pharmaceuticals. |
The past few years have seen a resurgence of dry eye exploration. With the Delphi and the International Dry Eye Workshop (DEWS) reports, our approach to diagnosing, treating, and managing dry eye has been revolutionized.
DEWS was an international panel of dry eye experts tasked to update our understanding of dry eye. The panel released several papers on definition and classification, diagnosis, epidemiology, treatment and management, and research. A fundamental change in our understanding of dry eye is evident in its current definition: “Dry eye is a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface. It is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface.” (DEWS Definition and Classification, 2007).
This new definition replaces our traditional definition, which was based on objective findings of aqueous deficiency or increased evaporation, to include patient symptoms and to incorporate the critical role that osmolarity, inflammation, and inflammatory mediators play in the disease’s initiation and perpetuation. With this new definition, dry eye disease doesn’t appear to be a broad enough term to incorporate all facets of the disease. Ocular surface disease (OSD) is a term beginning to appear in literature and continuing education meetings and may be a more accurate representation of this multifactorial condition.
Risk Factors
Women are the primary sufferers of dry eye, particularly older women. Schaumberg et al (2003) reported that the prevalence of dry eye disease increased with age from 5.7 percent in women younger than 50 to 9.8 percent in women older than 75. This translates into more than 3 million women older than age 50 in the United States who suffer from dry eye. In men, prevalence also increased with age from 3.9 percent among men 50 to 54 to 7.67 percent among men older than 80 (Schaumberg et al, 2009). Dry eye disease is common in patients who have autoimmune diseases, affecting approximately 8 percent of the population (mostly female) (Gayton, 2009). The most likely groups to be identified with dry eye disease are postmenopausal women and the elderly, but it’s important to recognize that it affects all ages. It is clear that sex hormones exert significant influence on disease development and may account for the difference in prevalence between the two sexes, which is discussed in greater detail below.
Other factors such as contact lens wear and LASIK—particularly in the several months after the surgery—are associated with dry eye. Activities such as computer use, television watching, and reading provoke dry eye symptoms. Climate and environmental challenges also are important considerations. Certain medications have a known association with dry eye, including antidepressants and antihistamines. The preservatives in many topical eye drops also increase ocular surface complications. Leung et al (2008) reported that patients using topical glaucoma medications had a significantly increased dry eye prevalence compared to non-glaucoma patients (16.5 percent versus 5.6 percent).
Etiological Classification of Dry Eye
The DEWS report reaffirmed the continued use of the two major groupings of dry eye: aqueous deficient dry eye (ADDE) and evaporative dry eye (EDE) (Figure 1). Aqueous deficiency can be subdivided into Sjögren’s syndrome dry eye and non-Sjögren’s dry eye. Evaporative dry eye may be secondary to intrinsic (abnormal lid function) or extrinsic (contact lens wear) factors.
In ADDE, the resulting dry eye is secondary to a failure in lacrimal tear production. The synthesis and presentation of normal tears to the ocular surface is controlled by the “lacrimal functional unit.” The lacrimal functional unit is composed of the lacrimal glands (both main and accessory), the ocular surface (cornea, conjunctiva, meibomian glands, and goblet cells), the muscles that control blinking, and the interconnecting innervation (Stern et al, 2004). The three major components of the tear film (mucin, aqueous, and lipid) are secreted onto the ocular surface in a coordinated fashion. This secretion results from subconscious stimulation of free nerve endings in the cornea generating afferent nerve pulses to the trigeminal ganglion via the ophthalmic branch of the trigeminal nerve. The impulses continue on to the pons where they integrate with other cortical and neural inputs. The efferent branch sends fibers through the pterygopalatine ganglion to the main and accessory lacrimal glands. If any portion of this unit is compromised, normal tear production is impeded. Normal tear production and secretion onto the corneal surface is crucial to prevent bacterial/viral infections, maintain ocular surface integrity, and to provide an optical surface for light refraction (Stern et al, 2004).
One of the important conclusions from the DEWS report is that tear hyperosmolarity is associated with damage to the ocular surface or inflammation in dry eye (DEWS Epidemiology, 2007). In ADDE, tear hyperosmolarity is a result of this reduced tear production from the lacrimal functional unit, although there is a normal evaporation rate. The increased tear osmolarity may damage the surface epithelium, which may trigger production of signaling molecules as well as decrease the number of conjunctival goblet cells. The inflammatory mediators produced include a variety of interleukins, tumor necrosis factor, and matrix metalloproteinases, which activate immature corneal dendritic cells. These antigen-loaded, antigen-presenting cells migrate from the ocular surface to the regional lymph nodes where they present antigen to CD4 T cells. Activated CD4 T cells migrate from the lymph nodes to the ocular surface and lacrimal glands where, if conditions are favorable, they infiltrate the tissue, and resulting cytokines produced by these CD4 cells modulate epithelial cell survival (Pflugfelder et al, 2008). Goblet cell loss by tear hyperosmolarity results in a mucin expression disturbance and tear film instability. The instability leads to tear film breakup and a resulting increase in ocular surface hyperosmolarity, perpetuating the inflammatory cycle (Zoukhri, 2006; Gaffney et al, 2010).
ADDE is subdivided into Sjögren’s and non-Sjögren’s dry eye (DEWS Definition and Classification, 2007). Sjögren’s syndrome, an autoimmune disease primarily affecting women (>90 percent), is a result of autoantibodies targeting exocrine glands such as the lacrimal and salivary glands. The lacrimal and salivary glands are infiltrated by activated T-cells, resulting in cell death and hyposecretion. There are two forms of Sjögren’s: primary and secondary. Primary has all the characteristics of Sjögren’s syndrome without a systemic autoimmune disease diagnosis. Secondary has all the characteristics of primary with a concurrent systemic autoimmune disease diagnosis such as rheumatoid arthritis (most common) or lupus (Sullivan, 2004).
Non-Sjögren’s dry eye occurs predominately in postmenopausal females and in women who are pregnant or taking estrogen-based birth control pills. Because most dry eye patients are women, estrogen was initially suspected as the key hormone involved. However, while postmenopausal women have diminished estrogen levels, pregnant women and those taking birth control pills have elevated levels. Also, evidence shows that women taking HRT, particularly estrogen alone therapy, have increased dry eye occurrence and meibomian gland dysfunction (Sullivan, 2004). Likewise, androgens are reduced in postmenopausal women due to decreased ovarian function and secretion of sex hormone binding globulin during pregnancy/birth control pill use (androgens also regulate meibomian gland and lacrimal tissue expression, Sullivan, 2004). Androgen support loss results in the ocular surface’s inability to respond appropriately when environmentally challenged (Stern, et al, 2004).
Other causes of non-Sjögren’s syndrome dry eye include secondary lacrimal gland dysfunction (secondary to sarcoidosis, AIDS), lacrimal gland duct obstruction (trachoma scarring), and reflex hyposecretion (reflex sensory block as seen in lens wearers and diabetics; reflex motor block as seen with damage to cranial nerve VII) (DEWS Definition and Classification, 2007).
EDE is a result of excessive tear evaporation from the ocular surface in the presence of normal secretion from the lacrimal functional unit. EDE can be further broken down into causes that are intrinsic and those that are extrinsic (Figure 1). Intrinsic causes include meibomian gland dysfunction (MGD), lid aperture disorders, and low blink rate. Extrinsic factors include ocular surface disorders (from vitamin A deficiency), contact lens wear, and ocular surface disease (allergic conjunctivitis) (DEWS Definition and Classification, 2007).
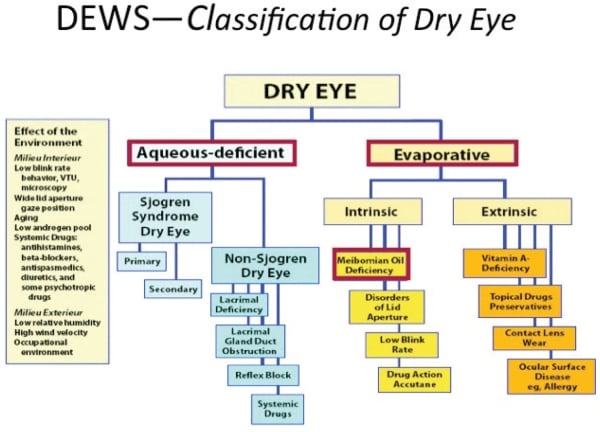
Figure 1. The DEWS Classification of dry eye.
Although we have a standard classification scheme for dry eye etiologies, we still don’t have a thorough understanding of how the different disease states progress. This progression could be different for each disease classification. For ADDE, possible subgroups could include: (1) mild/mild-to-moderate, which is characterized by lacrimal dysfunction, low aqueous secretion, and normal evaporation or (2) severe, associated with significantly decreased tear production (inadequate volume) and ocular surface desiccation. In the severe form, you could confuse this aqueous deficiency as an evaporative problem, although there could be overlap.
With EDE, possible subgroups could be: (1) mild/ mild-to-moderate, with an increased evaporation rate in the presence of a normal lacrimal functional unit; (2) mild-to-moderate, with greater evaporation and an increased lacrimal functional unit output due to compensatory increase in reflex tearing; and (3) severe, in which the underlying condition such as MGD is accompanied by a progressive reduction in lacrimal functional unit output. This is likely due to corneal damage and reflex sensory stimulation loss or lacrimal gland infiltration. This stage could be confused with an aqueous deficient dry eye secondary to the decreased aqueous production. It is likely that each form of dry eye goes through a natural progression through various stages of severity and, with that, there may be significant overlap between the two forms (Bron et al, 2009).
Dry Eye Diagnosis
The precorneal tear film is comprised of a superficial lipid layer that covers a middle aqueous layer of hydrated mucus gel containing soluble antimicrobial proteins and growth factors. Abnormalities in either layer can lead to dry eye. A combination of various subjective and objective measurements is commonly used to determine the presence and/or severity of an individual’s dry eye (Moore et al, 2009).
Traditional tests used to diagnose dry eye include the Schirmer test, tear-film breakup time (TBUT), and ocular surface staining utilizing sodium fluorescein, rose bengal, or lissamine green. Most clinicians utilize these tests, although they do have disadvantages. In some patients who have dry eye (particularly mild/moderate), these diagnostic tests can disagree or give conflicting results (Moore, et al 2009). These tests are invasive, making it difficult to acquire information about the natural state. Ocular surface staining tends to be subjective and qualitative and does not always correlate with dry eye symptoms. Studies have shown a poor correlation between Schirmer’s test and TBUT (Moore et al, 2009).
Noninvasive diagnostic techniques have been developed including quantitative assessment of tear volume, tear film stability, tear dynamics, and integrity of the ocular surface. Phenol red thread test (Zone-Quick) evaluates aqueous deficiency. It uses a thin thread infused with phenol red that becomes red when it comes in contact with tears. The thread is placed in the lower cul-de-sac without anesthetic for 15 seconds. Values of <10mm wetting have been indicative of aqueous deficiency (Moore et al, 2009).
The tear meniscus functions as a reservoir for tears and as a route for tear drainage and tear distribution. The tear meniscus contains 75 percent to 90 percent of the aqueous tear volume. The tear meniscus radius can be used as an index of tear volume over the ocular surface. The tear meniscus radius was found to be significantly smaller in dry eyes than in normal eyes. Video meniscometry has been used to noninvasively detect the height of the tear meniscus (Yokoi et al, 2004; Yokoi and Komuro, 2004).
Other assessments of the lids, meibomian glands, and lipid layer are critical in the evaluation of EDE, and are discussed extensively elsewhere in this issue (see Research Review).
Impression cytology is a technique for collecting conjunctival epithelial cells for analysis of ocular surface disorders. Three main cells can be found in impression cytology specimens: epithelial cells, goblet cells, and inflammatory cells. Analyzing these different cell populations, their shape, number, density and pathologic modifications provides valuable information pertaining to ocular surface status. For example, absence of goblet cells from conjunctival impressions is the hallmark of dry eye syndromes, whereas the presence of goblet cells in impressions obtained from the corneal surface is pathognomonic of conjunctivalization associated with stem cell deficiency. The presence of certain inflammatory cells can be used as markers for dry eye disease (Brignole Baudouin et al, 2004).
Measuring tear film osmolarity has traditionally been limited to research laboratories. The TearLab Osmolarity System (TearLab Corporation) is a recently commercially available micro-osmometer that measures osmolarity of tears obtained from the interior lateral meniscus. An independent study of the TearLab system demonstrated that it was able to accurately measure tear osmolarity with the same accuracy as a laboratory osmometer (correlation of 95 percent) (Tomlinson et al, 2006). The study also demonstrated ease of use. Study collectors, who read only the accompanying instructions and had no additional training, obtained samples that were 100 percent successful. No conjunctival or corneal trauma was noted in any of the subjects.
Adam Keech, OD, a research optometrist at the University of Waterloo School of Optometry, recently compared the tear osmolarity in dry eye disease patients (ocular surface disease index score >20) and in a comparable group of controls. Dr. Keech found that the dry eye patients had a significantly higher degree of osmolarity compared to the controls (Rajecki, 2010).
Treatment and Management
Treating and managing dry eye disease has traditionally been a trial and error process, often utilizing artificial tears as a mainstay “cure all.” It wasn’t until the Delphi panel report that a more evidence-based and systematic approach to treatment was proposed (Behrens et al, 2006). The Delphi panel recommended that practitioners first assess patients for lid disease and address any existing lid disease issues. For patients who don’t have significant lid disease and primarily suffer from an aqueous deficient disease, the associated treatment and management is recommended based on the severity of symptoms and ocular signs (for more in-depth information on blepharitis treatments see “Current Findings on Blepharitis,” article of this issue). The Delphi panel formulated a four-tiered progression of aqueous deficient disease and associated treatment protocols (Table 1).
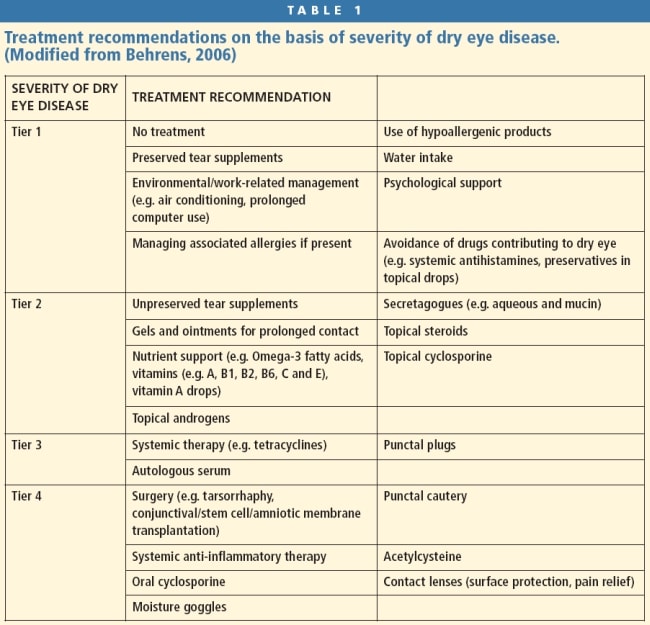
Tier 1 Patients in tier 1 severity have mild-tomoderate symptoms with/without mild-to-moderate conjunctival signs (hyperemia). For these patients, lifestyle measures are often useful and effective. Educate patients about increased symptoms with prolonged reading or computer use secondary to increased exposure and decreased blink rates.
Artificial tears are recommended to help alleviate patient symptoms and are particularly useful prior to, concurrently with, and after any prolonged near activity. Tear supplements provide temporary, palliative relief of symptoms although long-term relief may not be satisfactory. Tear supplements primarily replace the volume of the tears although they do not replace the other normal tear components that are necessary for a healthy ocular surface. Tear supplements are available in a variety of formulations and act on components of dry eye pathophysiology.
Preservatives continue to pose a significant concern for a healthy ocular surface. Several studies have demonstrated that patients who are on chronic glaucoma therapy have a significantly increased prevalence of dry eye disease (Leung et al, 2008). Recommend nonpreserved tear supplements for patients who instill them four or more times a day. Supplements are available in a variety of formulations with varying viscosity and electrolyte concentrations, which modify ocular surface pH and osmolarity (critical components in the inflammatory cascade). Long-lasting ointments are generally applied at night before sleep due to their effects on vision. Lacrisert (Aton Pharma) ocular inserts are slow-release ocular hydroxypropyl cellulose rods intended to reduce the need for frequent instillation of artificial tears (DEWS Management and Therapy, 2007; Luchs, 2008; Jackson, 2009).
Tier 2 patients have moderate-to-severe symptoms, tear film changes, and mild corneal and conjunctival staining with visual signs. The panel determined that these patients likely have an inflammatory component. Topical corticosteroids have a rapid onset of action and can produce dramatic clinical improvement, although they act in a non-specific, broad-spectrum manner. It is well-documented that long-term use of corticosteroids has the potential for significant ocular complications.
Cyclosporine A (Restasis, Allergan) has demonstrated good efficacy in reducing ocular surface inflammatory mediators and in improving patient tear production. Cyclosporine A can be used long-term in patients without significant side effects (DEWS Management and Therapy, 2007). The Delphi panel recommended that practitioners start patients on a topical corticosteroid (Lotemax, Bausch + Lomb) three to four times a day for two weeks prior to initiating Restasis (b.i.d. dosing). Then, patients should use the two drops concurrently for two to three weeks before tapering off the corticosteroid and maintaining Restasis long-term (Behrens, et al, 2006).
EyeRx Research, Inc., is developing a recombinant protein technology centered around the lacritin protein in human tears. Lacritin is thought to increase tear secretion and control underlying inflammation. The drop is currently in pre-clinical trials.
Secretagogues should also be considered at this stage. Secretagogues stimulate aqueous production or mucous secretion or both. Aqueous secretagogues include Diquas Ophthalmic Solution 3% (diquafosol tetrasodium, [Santen Pharmaceutical Co., Ltd.]), which was recently approved in Japan, and pilocarpine, although pilocarpine appears to be more effective for dry mouth than for dry eye symptoms.
Mucin secretagogues are in development. MIMD3 (Mimetogen Pharmaceutical) is a small-molecule mimetic of nerve growth factor, which has been found to stimulate mucin production in conjunctival goblet cells and to significantly improve clinical signs of dry eye in rodent models. Clinical trials started in 2009. Ista Pharmaceuticals is developing ecabet sodium, a mucin secretagogue, that increases the quantity and quality of mucin produced by conjunctival goblet cells and corneal epithelia.
Viva Drops (Vision Pharmaceuticals), a relatively new tear supplement, has shown good response with improved patient symptoms, decreased corneal staining, improved TBUT, and impression cytology analysis (Kim et al, 2009). Viva Drops contain retinyl palmitate, a form of vitamin A. Vitamin A is essential for maintaining the health of the epithelial and goblet cells.
Recommend that patients increase their intake of omega-3 fatty acids to help reduce inflammatory mediator production and that they reduce intake of omega-6 fatty acids. Several studies have demonstrated that consuming oral antioxidants, vitamins, and trace minerals significantly increases tear stability (Roncone et al, 2010). Vitamins A, B1, B2, B6, C, and E with trace elements including calcium, iron, and manganese can increase tear film stability in patients who have dry eye disease.
Androgens play a crucial role in dry eye disease. ArGentis is currently developing three hormonebased transdermal creams/gels. ARG101(T) is a testosterone cream or gel for menopausal women, ARG102(P) is a progesterone cream or gel for men and younger women, and ARG103 is a combination progesterone/testosterone cream or gel for a wider audience. These compounds are currently in clinical trials.
Tier 3 patients have severe symptoms, marked corneal punctate staining, central corneal staining, and possibly filamentary keratitis. In these patients, the inflammatory mediators have not been adequately controlled with tier 2 immunomodulators. A course of oral tetracyclines is recommended with the possible addition of punctal occlusion (Behrens, 2006). The tetracycline antibiotics have anti-inflammatory properties and decreased inflammatory mediators such as cytokines and matrix metalloproteinases (MMP-9). These inflammatory mediators are instrumental in the inflammatory cascade of aqueous deficient dry eye (DEWS Management and Therapy, 2007). Punctal occlusion lets patients retain more of their natural tears on the ocular surface. However, if residual inflammatory mediators are present, punctal occlusion can exacerbate the inflammatory process by allowing inflammatory mediators to remain on the ocular surface longer. The panel’s recommendation was to address the inflammatory component prior to initiating occlusion (Behrens, et al, 2006).
Autologous serum tears are also indicated for these patients. Autologous serum contains many of the components involved in the proliferation, migration, and differentiation of the ocular surface epithelial cells. The serum’s characteristics are similar to those of natural tears including pH and osmolarity, and it contains growth factors such as epithelial growth factor and platelet derived growth factor. Additionally, serum contains a variety of other nutritional factors necessary to maintain cellular viability. The serum is prepared using blood extracted from the patient and stored in vacuum extraction tubes without anticoagulant. The tubes are left to coagulate for a few hours and the resulting supernatant is centrifuged to separate out the serum. The serum is then bottled, typically at a concentration of 20 percent, and kept in a light-proof container to prevent degradation of certain components (vitamin A). Significant improvement in symptoms, TBUT, and staining have been reported in patients utilizing autologous serum drops. This is typically reserved for managing patients who have severe symptoms (Quinto et al, 2008; Kojima et al, 2008).
RegeneRx is developing RGN-259, a T4-based sterile eye drop, as a novel treatment for corneal healing. Preclinical studies have identified cell migration, anti-apoptotic and anti-inflammatory activities of T4 in the cornea. Resolvyx Pharmaceuticals is developing resolvin, RX-10001, a naturally occurring small molecule lipid mediator. As an active metabolite of the omega-3 fatty acid EPA, RX-10001 acts to protect healthy tissue during an inflammatory response to an environmental insult and to resolve inflammation once the environmental insult has passed. It is currently in clinical trials.
Tier 4 patients have tried and failed with all of the previous treatments, at which point surgical and systemic therapies are considered. Initiate systemic antiinflammatory therapy to control systemic inflammation and consider the potential of immunosuppressive agents (DEWS Management and Therapy, 2007). Surgical procedures suitable to treat severe dry eye include lid procedures (permanent punctal closure and tarsorrhaphy) and conjunctival procedures (conjunctival transplantation, amniotic membrane transplant, free conjunctival graft, and stem cell transplantation). Patients may also wear moisture-retaining goggles or scleral lenses. The lenses act as a reservoir for lubrication, serve as a mechanical barrier that protects the traumatized cornea from the blink trauma, and act as a “bandage” to help healing (Jackson, 2009).
Summary
Dry eye disease is a commonly encountered in practice. We must use a thorough case history of symptoms in conjunction with a variety of clinical signs and diagnostic tests to diagnose the condition and its severity. Ocular surface inflammation appears to be a key component in the initiation and perpetuation of the disease. Approach management and treatment options in a systematic way based upon symptoms and clinical signs of severity. CLS
For references, please visit www.clspectrum.com/references.asp and click on document #176.



