GP LENS BENEFITS
GP Versus Soft Lenses: Is One Safer?
When weighing the benefits to your patients and practice, one modality performs better.
By Thomas G. Quinn, OD, MS, FAAO (Dipl.)

|
Dr. Quinn is in group practice in Athens, Ohio. He is an advisor to the GP Lens Institute and an area manager for Vision Source. He has been an advisor or consultant to Alcon, B +L, Ciba Vision, CooperVision, and Vistakon, has received research funding from Alcon, AMO, B +L, Ciba, CooperVision, and Vistakon, and has received lecture or authorship honoraria from Alcon, AMO, B +L, CooperVision, GPLI, SynergEyes, and Vistakon. You can reach him at tquinn5@columbus.rr.com. |
Contact lenses offer a wide array of benefits to those in need of visual correction. This assertion is supported by the estimated $6.1 billion worldwide contact lens market (Nichols, 2011 Jan.), representing an estimated 120 million lens wearers (Nichols, 2011 Dec.).
Although among the safest forms of visual correction, contact lenses can sometimes result in health-related ocular complications. These complications can be reduced by employing a variety of strategies, which include improving patient compliance, encouraging proper use of new and improved care systems, better lens case care, and fitting more patients with GP lenses.
A Look at the Numbers
Well known for their exceptional visual performance, GP lenses, also referred to as gas permeable or rigid gas permeable (RGP) lenses, are often not immediately associated with enhanced safety. They should be. The proof is in the numbers.
Although the rate of microbial keratitis (MK) among all contact lens wearers averages only 4 per 10,000 wearers, the lowest rate of infections occurs in patients wearing GP contact lenses (1.2 in 10,000 wearers per year) (Stapleton et al, 2008). Is this a significant difference from the overall average rate?
When extrapolated out to include all contact lens wearers worldwide, if all were wearing GP lenses there would be 33,600 fewer wearers that would suffer the painful and vision-threatening risk of MK.
A study conducted in Australia and New Zealand found the median direct treatment cost for MK to be $760 with indirect costs of $468, totaling $1,228 per case (Keay et al, 2006 Oct.). In a theoretical world in which all wearers wore GP lenses, this adds up to a potential cost savings of more than $41 million. As per capita healthcare spending has grown more rapidly compared to per capita GDP for most of the past four decades (U.S. Senate Committee on the Budget, 2008), we can only conclude this number has grown significantly in year 2012 dollars.
Water, Water, Everywhere
One of the most serious corneal infections and most expensive to treat is Acanthamoeba keratitis (Keay, 2006 Jan.). Exposure of soft lenses and lens cases to simple tap water has been shown to increase the risk of Acanthamoeba infection (Mutoh et al, 2010; Tanhehco and Colby, 2010; Qian et al, 2010). What about GP lens exposure to water? Does that increase risk of infection?
Whereas the water content of hydrogel lenses ranges from 38 percent to 70 percent and that of silicone hydrogel (SiHy) lenses from 24 percent to 48 percent, GP lens materials contain less than 1 percent water (bausch.com; paragonvision.com). Microorganisms thrive in a warm, moist environment. The lack of water in GP lenses may help reduce survival of unwanted organisms such as Acanthamoeba.This notion is supported by studies that have found less adherence of Acanthamoeba to rigid lenses compared to soft lenses (Kilvington and Larkin, 1990).However, others have found at least one type of Acanthamoeba (A. castellanii), in its trophozoite form, to have greater adherence to GP lenses compared with soft lenses (Kelly et al, 1995). This effect can be somewhat mitigated by thorough lens rinsing. Cancrini et al (1998) found that rinsing in saline was much more effective at removing trophozoites from GP lens surfaces compared to soft lenses.
Regarding bacterial infection, it has been shown that swimming in both a chlorinated pool and in salt water while wearing hydrogel and SiHy contact lenses increases bacterial adhesion to the contact lens surface (Choo et al, 2005; Wu et al, 2011). This increases the risk of corneal infection (Keay et al, 2001). Hydrophobic and protein-laden surfaces bind more bacteria (Subbaraman et al, 2011), so a clean, hydrophilic surface will help reduce risk for any type of contact lens.
It is advisable for all contact lens wearers participating in water sports to wear goggles to reduce exposure to potential pathogens (Choo et al, 2005; Wu et al, 2011).It is also recommended that these wearers remove their lenses prior to sleep after having been exposed to water (Stapleton et al, 2007).
Corneal Inflammatory Events
While MK is a sight-threatening condition and certainly worthy of attention, corneal inflammatory events (CIEs) are more commonly encountered in clinical contact lens practice. CIEs include infiltrative keratitis, contact lens peripheral ulcers (CLPUs), and contact-lens-associated red eye (CLARE). One study showed that CIEs are thought to represent approximately 34 percent of all adverse events associated with soft contact lens wear (Wagner et al, 2011). Efron et al (2005) found GP lenses to have the lowest incidence of CIEs when compared to hydrogel and SiHy lenses.
A study comparing performance of high-oxygen GP lenses to soft lenses, both worn on a 30-day continuous basis, found nearly all soft lens adverse events to be inflammatory in nature (Morgan et al, 2005). There were significantly fewer adverse events overall for the GP lens wearing group, with only one inflammatory event.
The etiology of CIEs is not yet fully understood, but sleeping in lenses is significantly (2.37 times) associated with their development (Chalmers et al, 2011). The occurrence of CIEs during extended wear has been associated with lens bacterial bioburden (Szczotka-Flynn et al, 2010).The higher the bacterial load on the lens surface, the greater the likelihood of a CIE. Bacteria has been found to bind to some silicone hydrogel contact lenses in higher levels than to their hydrogel counterparts (Subbaraman et al, 2011), perhaps explaining the two-fold higher incidence of CIEs with silicone hydrogel lens wear over hydrogel lens wear (Szczotka-Flynn and Diaz, 2007; Radford et al, 2009).
Other studies have explored the effects of lens material on bacterial binding to the ocular surface. P. aeruginosa (PA), the most common organism associated with corneal infection (Green et al, 2008), has been found to bind to corneal epithelial cells at increased levels with wear of both traditional hydrogel lenses and silicone hydrogels. However, high-oxygen GP lenses did not significantly increase PA binding when worn on a daily wear or overnight wear basis (Ladage et al, 2001; Cavanagh et al, 2003).
There is some evidence that CIEs can result from interaction between some care systems and SiHy lenses (Carnt et al, 2009). Figure 1 shows corneal infiltrates that cleared when the patient was switched from a multipurpose care system to a hydrogen peroxide- based care system. This issue is rarely observed with GP lenses and care systems.
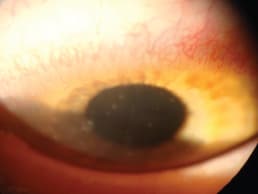
Figure 1. Corneal infiltrates from soft contact lens/solution interaction.
Importance of Tears on Corneal Health
Some have hypothesized that the development of corneal infections and CIEs may be associated with stasis of the tear film beneath the contact lens (Efron et al, 2005; Fleiszig and Evans, 2010). Tear exchange under the lens is thought to reduce risk of infection or inflammation by removing, or washing away, microbes from under the lens (Fleiszig and Evans, 2010; Polse, 1979).
Tear exchange under a GP lens is 10 percent to 20 percent, versus 1 percent to 2 percent tear replenishment rate per blink with soft contact lens wear (Polse, 1979; McNamara et al, 1999).
Tear film thickness beneath SiHy lenses has been found to be 2µm, thinning further with eyelid closure (Nichols and King-Smith, 2003; Qi et al, 2010). Tear film thickness under a GP lens varies with the base curve radius-cornea relationship, but is generally significantly greater: 8µm to 20µm have been used as working values (Taylor and Wilson, 1995). The presence of an adequate tear film beneath a lens allows tear components to act directly on microbes to compromise their integrity. Tear components have also been found to up regulate the defense capacity of corneal epithelial cells (Fleiszig and Evans, 2010).
The 10-times-greater tear exchange rate and generous tear reservoir found beneath most GP contact lenses likely provides a significantly superior protective effect over what exists during soft contact lens wear.
What About the Limbus?
All currently available soft contact lenses rest on and cross over the limbus. This is a vital area for corneal health as rejuvenating stem cells reside here (Schermer et al, 1986).
Dumbleton et al (2001) clearly demonstrated that hypoxia induced by low-oxygen-transmissible hydrogel lenses can result in limbal changes in the form of neovascularization (Figure 2).

Figure 2. Corneal neovascularization associated with soft lens wear.
Recently, Maissa et al (2012) also established that soft lenses can disturb the integrity of tissue in the limbal region by mechanical interaction between the lens periphery and the ocular surface (Figures 3 and 4). Lens edge profile and material rigidity are thought to determine the severity of disturbance. What impact this may have on stem cells is not clearly understood.
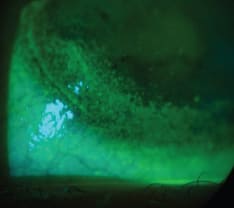
Figure 3. Area of corneal disturbance adjacent to the limbus on a soft lens wearer.
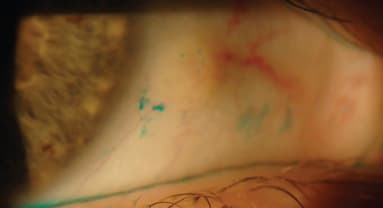
Figure 4. Area of conjunctival disturbance adjacent to the limbus on a soft lens wearer.
Corneal GP lenses do not generally interact with the ocular surface in the limbal region and are therefore unlikely to adversely affect tissue in this important area of the ocular surface, depending on the actual fit of the GP lens.
The Superior Tarsal Plate
Contact lens-induced papillary conjunctivitis (CLPC) can occur with wear of both soft and GP lenses (Figure 5).However, Forister et al (2009) found it to occur three times more frequently in soft lens wearers. The same study found complication rate overall to be significantly lower for GP lens wearers than for soft lens wearers (0.54 versus 0.85).
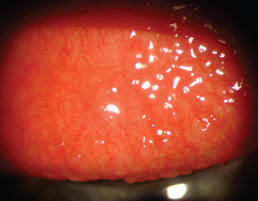
Figure 5. CLPC from wear of a silicone hydrogel lens.
Under-Utilized Resource
GP lenses account for only 9 percent of the worldwide contact lens market (Morgan et al, 2010). With their widely recognized visual benefits over soft lenses and the aforementioned safety profile, why are so few people being fit with these lenses? I believe it comes down to two issues: concerns about comfort and lack of confidence in fitting GP lenses.
Concerns About Comfort
Many patients, as well as practitioners, have the perception that GP contact lenses are not comfortable. Admittedly, some adaptation is required initially.
Employing anesthetic during initial lens application can serve to positively predispose patients to GP lenses. Bennett et al (1998 Nov.) found that this strategy improved initial comfort, significantly reduced dropouts, and enhanced perception of the adaptation process.
There is evidence suggesting that overnight lens wear may accelerate the adaptation process. Maldonado-Codina et al (2005) found that following overnight lens wear, neophyte GP lens wearers reported no difference in comfort compared to soft contact lens wearers or fully adapted GP lens wearers. Of course, this strategy should only be employed when fitting highly oxygen-permeable GP lenses.
Advances in GP lens fabrication allow manufacturers to produce thinner lenses with smoother surfaces and well-finished edges, all of which serve to promote comfortable wear. Specific recommendations in fitting approaches to enhance comfort will be reviewed later in this article.
It's All About the Presentation
Another key component to achieving successful adaptation to GP lens wear is to present this option in a positive manner.
Many patients either aren't aware of GP contact lenses or they associate them with hard (PMMA) contact lenses. Some education and orientation about the features and benefits of GP lenses is required to get patients properly receptive to the idea of pursuing GP lens correction. It has been demonstrated that patients are more likely to succeed with GP contact lens wear if the option is presented with genuine interest and a positive and realistic attitude (Bennett et al, 1998 July). Finding the right balance between over-promising and being negative can be challenging. See “GP Presentation Pearls” on page 58 for some key points to offering a positive, yet realistic, presentation.
Fitting Tips
As with soft contact lenses, proper lens centration and movement are the secrets to success. Evaluating the fluorescein pattern can be helpful in understanding what changes must be made to achieve the desired fit, but should not distract you from what is most important: lens centration and movement.
Start With Diameter
Begin by observing the location of the upper eyelid relative to the upper limbus. If the eyelid covers the upper limbus, which it does in most cases, choose a lens diameter that will allow the lens to tuck under the upper eyelid. This will usually be a diameter larger than 9.0mm, unless the patient has an unusually small palpebral aperture and horizontal visible iris diameter (HVID). Referred to as lid attachment, this approach helps stabilize the lens along the line of vision, enhancing visual performance. It's important to note that due to differences in lid tension, lid attachment may not perform as well on an Asian eye, in which case a smaller-diameter lens may perform better (Hom and Bruce, 2000).
Lid attachment offers the additional benefit of less initial lens awareness as the eyelid does not need to blink over the lens edge, as is necessary with lenses that reside within the palpebral aperture (Figures 6 and 7).
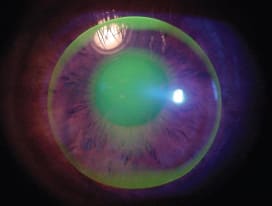
Figure 6. An interpalpebral lens fit, requiring the lid to blink over the lens edge.

Figure 7. A lid attachment fit with the upper lens edge tucked under the upper eyelid.
Three o'clock and 9 o'clock staining is among the most common adverse physiological events observed with GP lens wear (Forister et al, 2009). It is observed most commonly with low-riding GP lenses, particularly ones that drop and don't move sufficiently with the blink (Figure 8). The 3 o'clock and 9 o'clock staining often leads to conjunctival injection and discomfort. Employing the lid attachment fitting approach helps avoid this common GP problem by “attaching” the lens to the upper eyelid, preventing it from dropping. Reducing lens center thickness and employing lenticular edge profiles can also help prevent inferior lens decentration.
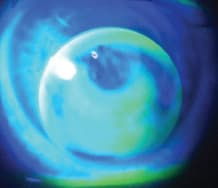
Figure 8. A low-riding GP lens.
Base Curve Selection
The goal is to align the horizontal central posterior lens surface with the underlying corneal surface. For large-diameter GP lenses (9.3mm to 10.2mm), choose a base curve that is 0.50D flatter than the flat keratometry reading. For smaller- diameter GP lenses, choose a steeper base curve radius.
GP Trends
Today's GP lenses are made of sophisticated materials that offer excellent oxygen transmissibility, good stability, and improved wetting performance over earlier generations. New manufacturing techniques produce lenses that are thin and light with smooth junctions and finely tapered edge contours. Lenses fit today tend to be larger and thinner than those fit in years past, providing better centration and movement on the eye and enhanced comfort (Laurent, 2009).
Some Personal Reflections
With all the concern about comfort with GP lenses, my personal experience has been that, after a number of hours of wear, my GP lenses are more comfortable than any soft lens I've worn. Soft lenses start to burn after hours of wear. I don't get that with my GP lenses.
I have, however, been frustrated struggling through episodes of transient foreign bodies getting under my lenses. These “contact attacks” are not serious, but they are annoying. I am pleased to report that this problem has been significantly reduced with wear of larger-diameter (10.0mm) GP lenses.
Finally, it has long been my impression that GP wearers who successfully overcome awareness associated with adaptation don't drop out of contact lens wear like soft contact lens wearers do. There is evidence that lends support to this impression (Morgan et al, 2005). I don't know if it's the type of patient personality, the effect soft contact lenses have on limbal stem cells, or some other phenomenon yet to be identified that gives these patients staying power, but GP wearers seem to be loyal, long-term lens wearers. So fit your patients with GP lenses. Provide exceptional vision, long-lasting satisfaction, and one of the safest forms of visual correction. CLS
For references, please visit www.clspectrum.com/references.asp and click on document #196.
| GP Presentation Pearls |
|---|
| 1. Rather than presenting a list of lens options, recommend GP lenses with confidence. 2. Prepare patients for the adaptation process by using the Sandwich Approach (necessary information communicated between two positive statements). OPENING POSITIVE STATEMENT: “I recommend we fit you with GP lenses. They will provide you with excellent vision, superior safety, and easy handling.” KEY MESSAGE: “There will be some initial awareness of the lens, much like adapting to a new watch or ring.” CLOSING POSITIVE STATEMENT: “GP lenses are a great fit for your needs.” 3. Practice the no-surprise approach to lens care. a. Make patients aware of the initial awareness with GP lenses, but don't dwell on it! b. Utilize anesthetic for initial lens application. Tell the patient, “I am going to put a drop in your eye to help you with the initial adjustment to the lenses. It will help me assess your vision and fit more accurately.” |



