NEWS spectrum
B+L to Acquire Ista Pharmaceuticals
Bausch + Lomb (B+L) has announced that it will acquire Ista Pharmaceuticals, Inc. in a transaction expected to be finalized in the second quarter of 2012. Under the terms of the transaction, which was unanimously approved by the boards of directors of both companies, B+L will purchase Ista for $9.10 per share in cash, or a total of approximately $500 million.
B+L officials said that the acquisition accelerates its strategy to strengthen its pipeline and marketed products and capabilities. Ista's portfolio includes nonsteroidal, anti-inflammatory, allergy, glaucoma and spreading agents, which B+L officials said would complement its existing prescription and over-the-counter eye health products. The companies also have complementary development pipelines. Ista's pipeline includes candidates in various stages of development to treat various ocular conditions including inflammation and pain, while B+L's pipeline includes the first of a new class of ocular anti-inflammatory agents and an approach to reducing intraocular pressure in patients who have open-angle glaucoma or ocular hypertension.
Brent Saunders, president and chief executive officer, B+L, said that because the company already manufactures nearly all of Ista's current U.S. products, the companies are well-known to each other and share a strong overlapping U.S. customer base.
Avaira Toric Receives Special 510(k) Clearance
■ CooperVision, Inc. recently announced that the U.S. Food and Drug Administration has granted a Special 510(k) clearance for the Avaira Toric two-week silicone hydrogel contact lenses for astigmatism. Cooper-Vision will re-launch Avaira Toric with shipments available for select distribution beginning in early May 2012.
CooperVision initiated a recall of select lots of Avaira Toric lenses on Aug. 19, 2011 because of the unintended presence of a residue on certain lots of Avaira Toric lenses, which may cause temporary hazy vision and discomfort. Following the initiation of the recall, the company received some additional complaints of severe eye pain. In November 2011, Contact Lens Spectrum reported that the recall had affected approximately 600,000 Avaira Toric lenses in the United States, according to CooperVision. In the fall, CooperVision expanded the recall to also include certain lots of Avaira Sphere lenses.
After thorough investigation, CooperVision identified the root cause of the condition and took the appropriate corrective actions, including manufacturing improvements, a new quality test, and increased quality standards. The company also put in place new metrics to track key quality parameters. All Avaira sphere and toric contact lens manufacturing lines are subjected to the additional quality controls put in place. A Cooper-Vision representative said the company is confident that these additional quality controls and manufacturing improvements rectified the manufacturing issue that occurred on limited lots of Avaira Sphere and Avaira Toric lenses.
CooperVision received the Special 510(k) clearance from the FDA to manufacture and resume shipping Avaira Toric lenses.
GPLI Announces Symposium in Denver
The Gas Permeable Lens Institute (GPLI) will host its next clinical symposium on June 3, 2012, in Denver (Lakewood), Col. The faculty for the event will feature some of the foremost GP lens experts in the United States including Dr. Christine Sindt, Dr. Tom Quinn, Dr. Shawna Vanderhoof, Dr. Perry Umlauf, Dr. Mindy Toabe, and GPLI Executive Director Dr. Ed Bennett.
Attendees can select from seven courses in both fundamental and advanced GP lens education, including specialty fits. For the first time, the GPLI will feature a live fitting demonstration within the scleral course offering. Attendees can earn up to seven hours of COPE credit, NCLE credit, and/or JCAHPO credit (approval pending).
The GPLI's “two track” approach to education allows practitioners and staff to select courses in Track One, pertaining to: (1) Spherical Fitting, Evaluation, and Troubleshooting, (2) GP Care and Compliance, and (3) GP Multi-Focals or Track Two, with presentations on (1) Toric GP Lenses, (2) GP Management of Keratoconus, and (3) Scleral Lens Fitting, Care, and Problem-Solving. Attendees can select courses from both tracks to meet their individual needs. All attendees will attend a preliminary GP update course as well as a closing Case Grand Rounds course with a series of cases reviewing a variety of GP patient types.
Attendees that select the Scleral Lens, Fitting, Care, and Problem-Solving course will be offered the opportunity to actively participate in a scleral lens fitting. The “workshop” approach featured in this course will include a live fitting demonstration to illustrate variations in fitting relationships when incorporating lens parameter changes. Participants will have the opportunity to practice application and removal of scleral lenses.
The cost to attend this all-day event is $250. Attendees that register before May 11 will receive a $50 early registration discount. Contact lens technicians/staff are offered a reduced registration rate of $175 and can also receive a $50 early registration discount for registering before May 11. For more information or to register, visit www.GPLI.info.
| CONTAMAC HONORED WITH QUEEN'S AWARD |
|---|
| Contamac has received The Queen's Award for Enterprise 2012 in the International Trade category. The award, well known as the United Kingdom's most prestigious for outstanding achievement, is given annually by Her Majesty The Queen for the highest levels of excellence. Contamac, which celebrates its 25th anniversary this year, has continued to grow in international trade. According to company officials, its international trade strategy is a complex series of relationships that include, but are not limited to, overseas distributors, a wholly owned subsidiary, a multilingual sales and marketing team, medical device experts, and strategic alliances. Integral to this strategy, research and development eyecare practitioner education is delivered through the company's Centre of Excellence and the recently formed Contamac Specialty Lens Institute. Robert McGregor, director of Contamac, said the company was proud of the recognition, and that it will continue to focus on providing exceptional products, professional services, and research and development to the global marketplace. |
European Ortho-k Meeting to Launch
The European Academy of Orthokeratology (EurOK), a non-profit, independent organization that represents the European section of the International Academy of Orthokeratology (IAO), will hold its inaugural meeting from June 9 to 10 in Madrid, Spain. International and European experts will provide two days of continuing education with simultaneous translation in Spanish and Italian. The exhibition will host the leading manufacturers in the field. Visit www.eurok.eu for more information.
IAO includes continental sections in America, Asia, Europe, and Oceania. It represents the interests and serves the needs of orthokeratologists worldwide. EurOK is a proponent of ortho-k in Europe, promoting this science to eye specialists and providing education in the field of corneal molding for refractive errors and the latest technology for myopia control.
| For the Record |
|---|
In the April issue on the cover and in the article “An Update on Specialty Contact Lens Research,” the photo attributed to Halina Manczak, MD, PhD, and described as “ClearKone in Optic Section View” was not the correct photo. That photo (left) was taken by Dr. Manczak but is an image of posterior keratoconus. The correct photo of ClearKone in optic section view by Dr. Manczak appears on the right. Contact Lens Spectrum regrets the error.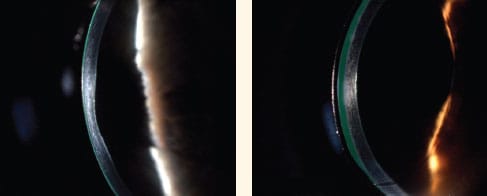
|
Remembering Dr. Adolph Lombart
Dr. Adolph Lombart, founder of Lombart Lenses, died December 28, 2011 at the age of 89.
Dr. Lombart was born on Oct. 3, 1922 in Norfolk, Va. After graduating from Virginia Tech and serving in the U.S Army, he enrolled at the Pennsylvania College of Optometry. Upon receiving his degree, Dr. Lombart moved back to Norfolk to start his private practice. A real innovator, he was the first optometrist to offer four-hour eyeglass service, something that was unheard of in the early '50s. It actually didn't become popular for another 30 years. He also opened multiple offices and had many optometrists on staff who later had their own successful practices.
In 1954, when he could not find a reliable source for contact lenses, Dr. Lombart started Lombart Lenses, a contact lens manufacturing facility that would supply him and his offices with high quality contact lenses that they didn't have to wait weeks to receive. He built the business into a leading company when his son Kenneth joined him, and together they became leaders in the industry.
After building the business into the largest hard contact lens manufacturer, Dr. Lombart sold the company in 1973 to American Sterilizer of Erie, Pa.; his sons Kenneth and Rick ultimately went on to start Lombart Instruments in 1979. Dr. Lombart retired in 1973 to play golf and fish, two of his favorite things other than his wife and children.
In 1979 after a few years in Florida, Dr. Lombart helped the new owners develop a soft contact lens, Amsof, which shook up the industry by bringing the cost down to a very affordable level. Shortly after that he retired a second time. Lombart Lenses was sold several more times after he retired before eventually becoming part of CooperVision.
Dr. Lombart is survived by his wife of 67 years Bette and his three children Kenneth, Kathy Kantor, and Richard as well as five grandchildren and three great grandchildren.
Based in part on an obituary from The Virginian-Pilot, Dec. 29, 2011.
| INDUSTRY BRIEFS |
|---|
| ■ Alden Optical, Inc. has announced that it will extend its series of NovaKone webinars through May 2012. The webinars cover lens design, patient selection, and fitting philosophy and are designed to ensure that practitioners are successful with this novel soft lens design for keratoconus. To register for a NovaKone Introductory Webinar, visit www.aldenoptical.com/novakone or contact Alden Optical at (800) 253-3669. ■ Visionary-Optics LLC has announced two new distributors of its Jupiter Scleral lenses in the U.S. market: ABB Concise and Universal Contact Lenses of Florida, Inc. ABB Concise's consultants may be contacted at the company's Marshfield, Mass. office at (800) 225-1812 or at the Alameda, Calif. office at (800) 772-3911. The consultant staff at Universal Contact Lenses of Florida may be reached at (800) 874-4884. ■ Menicon Co., Ltd.'s Magic, the world's thinnest daily disposable contact lens, won a Gold Award in the Design Lotus category at the Asia Pacific Advertising Festival (ADFEST). In addition to the Gold Award for best logo, package design, and overall communication plan, Magic also won three Silver Awards. Magic's novel flat pack, created through Menicon's proprietary technology, contains a uniquely prepared contact lens and measures a thickness of just 1mm. Magic was launched on a pilot basis at the company's Magic Store Tokyo concept shop in Japan on Nov. 7, 2011, and will be sold nationwide in Japan beginning this June. ■ Research company GfK Malaysia has found that Malaysia's cosmetic contact lens market is rapidly growing. The company's latest findings indicate that consumers in Malaysia spent nearly US $14 million or 28 percent more on this segment in 2011 compared to the previous year. The company believes this is due in part to the easily accessible and widely available inexpensive cosmetic lenses offered by a growing number of manufacturers in the market, with the fastest growing segment being quarterly replacement lenses that are more affordable. ■ CooperVision has increased its partnership commitment to Optometry Giving Sight (OGS) by becoming a Platinum Global Sponsor starting Jan. 1, 2012. The company had made a two-year commitment to OGS and had announced a Gold Global Sponsorship last year. CooperVision customers also welcomed the partnership by making OGS their International Charity of Choice. ■ Researchers at the University of Tokyo's School of Medicine have shown that caffeine intake can significantly increase the eye's ability to produce tears. All of the 78 participants in the new study produced significantly more tears after consuming caffeine than after taking a placebo. The study subjects were divided into two groups: one received caffeine tablets in the first session and a placebo in the second session, while the order was reversed for the other group. No subjects knew whether they received the caffeine or the placebo. The study is available in the journal Ophthalmology. ■ FocalCenter recently launched www.focalcenter.com, an information platform for EyecareScore—FocalCenter's standardized feedback solution for eyecare, eyewear, and managed care providers. The site aims to inform users of the features, benefits, and effectiveness of EyecareScore. The company says that visitors will gain an understanding of the rationale for use, relevance to their business and robustness of this tool. ■ The Optometric Historical Society will hold its annual Reminisce-IN honoring optometry's history and heritage during Optometry's meeting in Chicago at 3 p.m. Thursday, June 28. The meeting will honor the life and contributions of Dr. Irvin Borish, who passed away on March 3, 2012. The tribute will be led by his long-time friend Dr. Alden N. Haffner, President Emeritus of SUNY, College of Optometry. ■ WebMD recently launched Eye TV for display within the Eye Health Center on webmd.com. Eye TV provides on-demand video programming that will bring to life real people successfully managing their eye health and vision, according to Web-MD. The company says that original, inspiring, timely, and informative video programming will celebrate the many faces of today's eye-health consumers and their families. |



