OCULAR WELLNESS
Maintaining Ocular Surface Wellness
A review of new and emerging examination techniques for diagnosis and management.
By Sruthi Srinivasan, PhD, BS Optom, FAAO
The term “ocular surface wellness” encompasses the health of many different structures of the eye including the eyelids, eyelashes, meibomian glands, tarsal and bulbar conjunctiva, cornea, and tear film, to list a few. The health of one structure can affect the health of others, which makes evaluation and management of ocular health quite difficult. There are many objective methods to evaluate ocular wellness in contact lens wearers. This article discusses several contemporary tests and techniques to assist eyecare practitioners with diagnosis and management of “ocular wellness” in contact lens and non-lens wearers.
Meibomian Gland Evaluator
The Meibomian Gland Evaluator (MGE), developed by Korb and Blackie (2008) and TearScience, is a handheld instrument used to evaluate meibomian gland (MG) function (Figure 1). The instrument provides a standardized method to apply consistent, gentle pressure at 1.25g/mm2 to one-third of the lower eyelid.

Figure 1. A Meibomian Gland Evaluator in use with standardized pressure being applied just below the lower lid margin. (Image provided by the CCLR, University of Waterloo.)
The presence of liquid secretion from a MG orifice (visualized through the slit lamp biomicroscope) during expression with the MGE indicates MG functionality (Korb and Blackie, 2008).
Mastrota Paddle to Evaluate Meibomian Gland Dysfunction
The Mastrota paddle is designed to gently express meibum from the MGs (Figure 2). The Mastrota paddle can be positioned behind the eyelid parallel to the glands. Gentle digital pressure, or pressure with a cotton-tipped applicator, on the outer lid prompts meibum secretion. This procedure can be carried out with or without anesthesia. This tool can be useful in examining MGs and grading MG function. (To view a video of a Mastrota paddle in use, click here.)

Figure 2. A Mastrota paddle placed on the inside of the lower eyelid. A cotton-tipped applicator is used to apply pressure on the outside of the lower lid (in the same position of the Mastrota paddle) to express the meibomian glands. (Image provided by the CCLR, University of Waterloo.)
MG Expressor Kit
The MG Expressor Kit from Gulden Ophthalmics was conceptualized and developed by Dr. Mario Gutierrez of San Antonio, Texas. The kit includes two gel eye masks, an ergonomic lid plate tool, a roller tool, and disposable expressor covers.
To start, the warmed eye mask helps liquefy the meibomian gland contents. Next, the MG Lid Plate is placed behind the lid, and then the tool is gently rolled over the eyelids and eyelid margins to help noninvasively express and decongest the meibomian glands (Figure 3). There is no need for topical anesthetics or cotton-tipped applicators, and the procedure can be easily performed with or without a slit lamp.

Figure 3. The MG Lid Plate is placed behind the lid and the tool is gently rolled over the eyelids to express and decongest the meibomian glands.
Forceps for Meibomian Gland Expression
One way to assess MG output is to use forceps to express some meibum. Several companies in the marketplace offer pointed and rounded forceps that can be used for just such a purpose. Products in this category include OcuSci’s Arita Meibomian Gland Expressor, Bruder Healthcare Company’s Collins Expressor Forceps, Rhein Medical Inc.’s Tearse Meibum Expressor Forceps, and E. Janach srl’s Alberti Meibomian Gland Expressor Forceps.
LipiView Interferometer
In a typical clinical setting, lid and lid margin evaluation is often performed using the slit lamp biomicroscope; however, the lipid layer cannot be clearly visualized with a slit lamp in isolation. The best option is to use a device such as an interferometer, which can view the lipid layer noninvasively.
The LipiView interferometer was developed by TearScience to evaluate the tear film in patients who have meibomian gland dysfunction (MGD) and dry eye in a clinical setting (Blackie et al, 2009). This instrument (Figure 4) requires only a few minutes to obtain an appropriate image of the tear film lipid layer from both eyes. Partial blinks can be assessed along with changes in lipid layer thickness (LLT) and appearance. It is also of value to image the tear film before and after the treatment of MGD.

Figure 4. The LipiView system can be used to evaluate the tear film lipid layer and blink patterns. (Image provided by the CCLR, University of Waterloo.)
It can be useful to evaluate the lipid layer and blink patterns in contact lens wearers using the LipiView system. Future studies to assess the use of the LipiView system in contact lens wearers who have MGD and dry eye are required.
Meibography
Although the above mentioned techniques allow visualization of the lids, lid margin, and lipid layer, they do not allow viewing of MG morphological changes. Meibography involves visualizing and photographing the morphology of MG structures in the tarsal plate. It is the only method available for assessing the number of partial glands or the total MG dropout assessment in the tarsal plate (Jester et al, 1982; Mathers et al, 1991; Mathers et al, 1994; Nichols et al, 2005). A recent study has shown that contact lens wear is associated with a decrease in the number of MGs, and this decrease is proportional to the duration of contact lens wear (Arita et al, 2009).
Historically, this methodology was regarded as more of a research procedure, but recently a number of noninvasive, clinician-friendly devices have been released (Arita et al, 2008; Arita et al, 2013). Several new devices that have appeared in the recent past, such as hand-held infrared (IR) meibography devices and slit lamps with IR illumination, aim to make meibography quicker and easier. These devices help with procedures used for examination and grading of MG dropout.
One example is the Keratograph 5M from Oculus, Inc., which is designed to provide a variety of non-contact corneal imaging as well as tear film and contact lens assessment tools. The IR illumination system of the Keratograph 5M (at 880nm) captures both video and still images of the MGs within the upper and lower lids when everted (Srinivasan et al, 2012) (Figure 5).
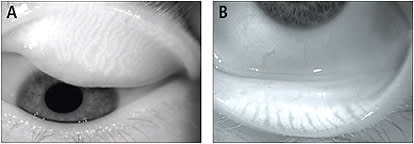
Figure 5. The meibomian glands in the upper eyelid (A) and in the lower eyelid (B) taken by the infrared camera of the Keratograph 5M. (Images provided by the CCLR, University of Waterloo.)
Osmometers
Tear hyperosmolarity is believed to be one of the core mechanisms that trigger the cascade of events in dry eye, such as ocular surface inflammation and symptoms of dryness (International Dry Eye WorkShop [DEWS], 2007; Farris et al, 1983; Lemp, 1995; Gilbrand et al, 1987). Increased osmolarity triggers ocular surface inflammatory events, which in turn causes tear film instability (DEWS, 2007). An unstable tear film may result in tear evaporation and increased tear osmolarity, which completes a vicious cycle that intensifies the disease.
A meta-analysis of published data on osmolarity (conducted in the past 25 years) has shown the cutoff value to be 316mOsm/L (Tomlinson et al, 2006). More recently, diagnosis of dry eye based on osmometry measurements has been utilized as a diagnostic tool, sometimes in combination with a battery of other dry eye tests.
The TearLab osmometer (TearLab Corp.) is a nanoliter instrument that offers a relatively expertise-free method for tear osmolarity measurement (Sullivan, 2005; Dalton and Jones, 2005). This “lab-on-a-chip” technology has enabled osmolarity measurement in clinical settings. This instrument determines the osmolarity by obtaining the electrical impedance of an electric current that is passed through the tear sample (Sullivan, 2005) (Figure 6).
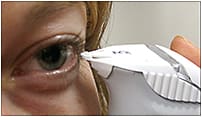
Figure 6. The TearLab osmometer uses a chip placed at the outer canthus of the eye to collect a tear sample. (Image provided by the CCLR, University of Waterloo, and TearLab Corp.)
Rapid Pathogen Screening (RPS) InflammaDry Detector
The RPS InflammaDry Detector (Rapid Pathogen Screening, Inc.) detects levels of matrix metalloproteinase-9, or MMP-9, in tears (Figure 7). MMP-9 is considered to be a marker for inflammation, usually associated with dry eye. InflammaDry is an in-office screening test that detects MMP-9, which is usually elevated in the tears of dry eye patients. This device uses direct sampling microfiltration technology to identify elevated levels of MMP-9 protein in tear fluid samples taken from the inferior palpebral conjunctiva.
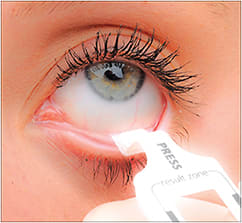
Figure 7. The RPS InflammaDry Detector tear collection device. (Image provided by Rapid Pathogen Screening.)
InflammaDry requires no additional equipment to administer or interpret results. Test results are achieved in approximately 10 minutes. The results of the test are indicated through two lines that appear in the result zone: the control line and the result line. A negative result is indicative of an MMP-9 level < 40ng/mL. A positive result indicates the presence of MMP-9 ≥ 40ng/mL.
A positive result is denoted by the presence of both a blue line in the control zone and a red line in the result zone. The presence of only a blue line in the control zone indicates a negative result.
TearScan MicroAssay System
The TearScan MicroAssay system (Advanced Tear Diagnostics, LLC) quantifies levels of lactoferrin (a dry-eye marker) and IgE (an allergy marker) in the tears. This tool helps to distinguish dry eye from allergic conditions.
A small sample of tears (0.5mL) is collected via a micropipette (Figure 8). The sample is placed in a diluent and then in a small well in a disposable cassette, which is introduced into the microassay unit. The microassay unit measures the amount of biomarker in the small tear sample. The test time is less than two minutes for lactoferrin and approximately five minutes for IgE.

Figure 8. With the TearScan MicroAssay System, a tear sample is collected with a micropipette.
In contact lens wearers, a low level of lactoferrin in tear samples is indicative of a possible risk of hypoxia, conjunctivitis, and/or dehydration. High levels may indicate excess contact lens deposition. A high level of IgE in contact lens wearers is indicative of possible risk of giant papillary conjunctivitis or inflammation (Zhao et al, 2008).
Optical Coherence Tomography
Optical coherence tomography (OCT) devices can help clinicians determine whether the tear volume is reduced by noninvasively measuring tear meniscus dimensions (Figure 9). OCT is also helpful in the visualization of conjunctival flaps or lid parallel conjunctival folds (LIPCOFs).

Figure 9. An optical coherence tomography image capturing the tear prism, cornea, and adjacent lower lid. Images can be taken on multiple visits to determine any change to the height of the tear prism and overall volume of the tear meniscus. (Image provided by the CCLR, University of Waterloo.)
Confocal Microscopy—Rostock Cornea Module on HRT III
More recently, Heidelberg Engineering GmbH developed a digital confocal laser scanning microscope, a combination of Heidelberg Retina Tomograph III and the Rostock cornea module (RCM). The RCM images cellular structures and can image the different layers of the entire cornea, from the epithelium to the endothelium. The RCM also enables the imaging of the peripheral areas of the cornea and conjunctiva (Figure 10). The instrument allows a focal range of up to 1,500µm with a field of view of 400µm x 400µm. This instrument offers three capture modes:

Figure 10. Confocal microscopy images of the cornea. The corneal nerves are visible in both scans. (Images provided by the CCLR, University of Waterloo.)
1) Section takes one image (i.e., a photograph).
2) Volumetric takes a depth scan of 80µm at 2µm resolution.
3) Sequence is a live capture (i.e., video capture).
The charge-coupled device (CCD) camera allows a permanent monitoring of the corneal contact on the screen. The RCM technology provides better image quality and produces a precise depth measurement compared with confocal-slit scanning microscopes.
Lid Debridement-Scaling
Debridement-scaling is a scaling procedure performed using a stainless steel golf club spud to remove any accumulated material on the line of Marx (LOM; line running along the inner eyelid) and keratinized lid margin (Figure 11). No anesthetic is required to conduct this procedure. Lissamine green dye is used to determine the location and thickness of the LOM (Korb and Blackie, 2013).
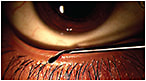
Figure 11. A stainless steel golf spud removing debris from the lid margin. (Image provided by the CCLR, University of Waterloo.)
A safety lens (a soft contact lens or a scleral lens) can be applied to protect the cornea in the case of a slip of the golf club spud during the debridement procedure. The debridement-scaling of the LOM and lower lid margin can provide significant symptom relief and improvement in the functionality of meibomian glands. Korb and Blackie (2013) have suggested this novel procedure to be considered in the management of MGD and evaporative dry eye.
Eyelid Care and Hygiene
There are a few new options for care of a patient’s eyelids relative to managing, or perhaps even preventing, ocular surface disease. BlephEx (Rysurg, LLC) is an in-office microblepharexfoliation procedure that allows eyecare practitioners to completely remove the debris, crusting, keratinization, and exotoxin-laden biofilm that builds up on patients’ lid margins. The BlephEx handpiece is used to spin a medical grade micro PVA sponge at 1,000rpm along the edge of the eyelids and lashes. This procedure lasts about six to eight minutes. It is typically repeated at four- to six-month intervals, sometimes sooner, as needed. A fresh tip is used for each eyelid. (To view a video of a BlephEx treatment being performed on a patient, click here.)
Another potential option for eyelid hygiene in ocular surface disease is Novabay Pharmaceutical’s Avenova. This product is FDA cleared as a medical device and is a prescription product. The company reports that two squirts of the product should be applied to a round cotton pad, and the pad should then be used to wipe the lids at the base of the upper and lower eyelid lashes twice daily for two weeks. According to the company, the product has “anti-microbial, anti-toxin, and anti-biofilm preservative activity in solution” and thus helps reduce eyelid contamination, and it improves eyelid hygiene in a two-week period.
A final option is OcuSoft HypoChlor, a 0.02% concentration of hypochlorous acid in both spray and gel formulation. According to OcuSoft, hypochlorous acid formulas do not contain the surfactants found in other eyelid hygiene products and thus are largely ineffective in debriding the oil, scales, and debris often associated with eyelid irritations. Accordingly, in the most severe cases, it is suggested to use combination therapy including both a surfactant cleanser and hypochlorous acid to achieve optimum results.
LipiFlow Thermal Pulsation System
The most common therapy for MGD is a combination of warm compresses, eyelid massage, and lid hygiene. These methods have been prescribed for decades without a standardized protocol. Significantly, the amount and duration of heat applied to the eyelid surface and duration of lid massage is inconsistent across studies and between treatment protocols (Mori et al, 2003; Ishida et al, 2008; Matsumoto et al, 2006).
The LipiFlow system (TearScience) is a thermal pulsation system believed to effectively relieve MG blockage, allowing the glands to resume the production of appropriate amounts of meibum. The in-office procedure takes approximately 12 minutes per eye (Friedland et al, 2011; Korb and Blackie, 2010).
This device has a single-use eyepiece that applies a controlled amount of heat and massage to the eyelids, treating the upper and lower lids simultaneously (Figure 12). The FDA-approved device is designed to treat MGD without damage to the glands or the surface of the eye. This procedure liquefies the MG contents and facilitates release of the gland’s secretion.
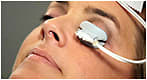
Figure 12. The LipiFlow thermal pulsation eyepiece placed on the eye of a patient.
Although contact lens wearers have been included in prior studies that have used the LipiFlow System, no study has assessed the treatment effects related to contact lens comfort. Prospective studies to assess the effects of this treatment in contact lens wearing patients who have MGD and dry eye are required.
MiBoFlo ThermoFlo Meibomian Duct Therapy
Another novel therapy aimed at improving function of the MGs is the MiBoFlo ThermoFlo Meibomian Duct Therapy (Pain Point Medical Systems Inc.). This device uses a thermoelectric heat pump to help liquefy pasty meibum, thus improving the tear film’s lipid layer.
The device supplies a continuous and controlled amount of heat that is applied to the outer skin of the eyelids with ultrasound gel for a gentle massage (Figure 13). The heat applied to the outer skin of the eyelids breaks down the solidified oils in the MGs.

Figure 13. The MiBoFlo ThermoFlo Meibomian Duct Therapy device placed on the eye of a patient.
The MiBoFlo has an adjustable timer, allowing treatment for various levels of dry eye. The protocol suggests an initial treatment for 12 minutes per eyelid, followed by a second treatment in two weeks for 10 to 12 minutes. A third treatment is typically given for eight to 10 minutes per eyelid. A fourth treatment may be required in some severe cases; whether that additional treatment is necessary should be determined during a follow-up evaluation visit.
This device is one of the recent entrants to this market, and further research is warranted in non-contact lens wearers and in lens wearers who have MGD and dry eye.
Summary
Ocular surface wellness can be affected by a number of variables, such as environment, systemic disease, and contact lens wear. Contact lens wear disrupts the tear film and may cause discomfort during wear. If changes to ocular wellness are not addressed, severe dry eye or contact lens dropout could result.
As mentioned above, various different tools are available to assess ocular wellness in contact lens patients. Not all devices will help improve a patient’s ocular wellness equally; thus, practitioners need to keep in mind what each device can offer. CLS
For references, please visit www.clspectrum.com/references and click on document #236.
This article was prepared with financial support from Alcon.

| Dr. Srinivasan is a research assistant professor and a clinical research manager at the Centre for Contact Lens Research, School of Optometry and Vision Science, University of Waterloo, Canada. She is a member of the Association for Research in Vision & Ophthalmology, the Tear Film & Ocular Surface Society, and the International Society for Contact Lens Research. She also serves as a Scientific Program Committee member of the American Academy of Optometry. |



