SCLERALS FOR OSD
Scleral Lenses for Severely Diseased Eyes
The eyes that can benefit the most from scleral lens wear are often the most difficult to fit.
By Karen Carrasquillo, OD, PhD, FAAO, & Gregory W. DeNaeyer, OD, FAAO
Severe ocular surface disease encompasses a variety of conditions that significantly affects a patient’s quality of life. This often prevents patients from driving, working, or carrying out any number of daily activities of living. Severe ocular surface disease is often secondary to a primary systemic condition, in which case a patient will have other health issues. These patients often are depressed and hopeless when traditional management strategies have failed.
Although not a cure, scleral lenses are often able to significantly reduce such patients’ symptoms and improve vision to the point that they can resume many activities that they had previously enjoyed.
Defining Severe Ocular Surface Disease
The ocular surface ecosystem is comprised of all of the anterior structures of the eye, including the cornea, conjunctiva, and the lacrimal and accessory glands. To have a stable and healthy ocular surface ecosystem, there must be good lid function, a healthy tear film, and a balance of growth factors and nutrients to enable/promote healing and sustainability. Any compromise to any or all of these structures will lead to ocular surface disease. Patients who have severe ocular surface disease fall within level 3 or 4 of the Dry Eye Workshop classification system of dry eye severity (Pflugfelder et al, 2007). Clinical signs and symptoms of level 3 include severe frequent or constant discomfort, visual limitations, moderate-to-significant conjunctival staining, marked corneal staining, and filamentary keratitis.
Clinical signs and symptoms of level 4 include disabling discomfort, disabling vision, marked conjunctival staining, severe corneal punctate erosions, filamentary keratitis, trichiasis, keratinization, and symblepharon (Pflugfelder et al, 2007). Compromised ocular surfaces, i.e., in cases of neurotrophic keratoconjunctivitis and limbal stem cell deficiency, will also exhibit poor healing capacity and/or conjunctivalization of the cornea by migration of conjunctival epithelium into the corneal surface. As mentioned, severe ocular surface disease often results from systemic conditions, some of which include graft-versus-host disease (GVHD), Stevens-Johnson syndrome (SJS), and Sjögren’s syndrome.
Ocular Surface Management
Patients who have severe ocular surface disease may often have multiple practitioners managing their care. These patients will be taking medications to treat their systemic condition and using maximum medical topical therapy, including artificial tears (drops and ointments), antibiotics, steroids, cyclosporine, and autologous serum tears. They may even undergo multiple surgical procedures, including amniotic membrane transplants, mucous membrane transplants, marginal lid rotation, punctal cautery, and penetrating keratoplasty.
The unfortunate reality is that despite systemic and ophthalmic intervention, many of these patients remain disabled secondary to reduced comfort and vision. However, scleral devices can often significantly improve their symptoms and visual function. By entirely vaulting the cornea and holding a liquid reservoir that provides continuous hydration to severely compromised surfaces, and by protecting the ocular surface from the harsh environment of damaged or transformed eyelids and from chronic exposure, these devices provide prosthetic replacement of the ocular surface system (PROSE). Prosthetic devices replace all or part of the function of a permanently inoperative or malfunctioning external body member or internal body organ; this is exactly what scleral devices are achieving when used for therapeutic purposes. Numerous reports have shown the prosthetic function of these devices (Theophanous et al, 2015; Cressey et al, 2012; Papakostas et al, 2015; Chui et al, 2014; and others; full list available at www.clspectrum.com/references.).
The following cases demonstrate the prosthetic use of scleral devices in patients who have severe ocular surface disease.
Case 1: Graft-Versus-Host Disease
In 2004, a 66-year-old Caucasian male had a history of GVHD secondary to stem cell transplant (September 2003) for acute myelogenous leukemia (diagnosed June 2003). He suffered from chronic persistent epithelial defects (PEDs) related to severe dry eyes from chronic GVHD, which eventually led to bilateral corneal melting and resulting penetrating keratoplasty in both eyes. He was referred in October 2004 for custom scleral devices with the goal to support the ocular surface moving forward. Medications at the time included prednisone p.o. 30mg/day, tacrolimus p.o. 4mg/day, Restasis (Allergan) 0.05% b.i.d. OD and OS, Tobradex (Alcon) ointment q.i.d. OD and OS, and Muro 128 (Bausch + Lomb [B+L]) 5% ointment q.i.d. OD and OS. He wore bandage lenses on both eyes and had medial and lateral tarsorrhaphies bilaterally.
The patient was fitted at the time with custom scleral devices in the following parameters that provided best-corrected vision of 20/40 in both eyes:
OD: Sag height 2600µm @ 12mm chord, base curve (BC) 8.2mm, –2.75D power, 18.5mm overall diameter (OAD), spherical haptics, Boston XO (B+L) material.
OS: Sag Height 2450µm @ 12mm chord, BC 7.9mm, –1.75D power, 18.5mm OAD, spherical haptics, Boston XO material.
The patient continued to do well with these devices, both with regard to relieving the severe dry eye symptoms associated with GVHD and for supporting the ocular surface to prevent further breakdowns. He was seen every one to two years for comprehensive follow-up evaluations while working in close collaboration with his local cornea specialist.
In June 2010, the patient developed an E. coli ulcer involving the temporal aspect of the corneal graft-host junction in the right eye. The infection cleared with treatment, but resulted in significant corneal scarring and thinning in the right eye; he required a repeat corneal transplant in the right eye. He subsequently developed another central PED. He was fitted with a 16.5mm diameter bandage contact lens, which decentered laterally. Hence, he was refitted into a 19.5mm bandage contact lens.
In September 2010, the patient’s cornea specialist referred him back to reassess customization of the scleral device in the right eye. Topical medications at the time included Pred Forte (Allergan) 1% q.i.d. OD and b.i.d. OS, Zymar (Allergan) 0.3% q.i.d. OD, timolol 0.5% b.i.d. OD and OS, and frequent lubrication. Systemic medications included prednisone, Septra (Pfizer), and doxycycline. There was consideration of a repeat graft or Descemet’s stripping endothelial keratoplasty (DSEK) in the left eye in the near future because of clinical signs of corneal thickening and development of epithelial edema at baseline (without wearing his scleral device) in the left eye.
At time of evaluation, best-corrected visual acuity (BCVA) OD was 20/200, pinhole (PH) no improvement (NI) with the bandage contact lens in place. The bandage contact lens had good movement and offered good corneal coverage, but decentered temporally. The size of the PED was ~1.5mm x 2.5mm (Figure 1).

Figure 1. A) Repeat corneal graft and, B) persistent epithelial defect after repeat corneal graft in the right eye.
Evaluation of the left eye, wearing a spherical custom scleral device, indicated that the ocular surface was supported, and no PED was noted; however, there were signs of epithelial microcystic corneal edema (MCE), corneal thickening, and subjective reports of “seeing rainbows” when looking at a light source, otherwise known as Sattler’s veil; this is indicative of fluid retention/edema in the cornea. The BCVA was 20/30+2, but vision blurred after one hour of device wear associated with MCE. Endothelial cell count in the left eye (measured with confocal microscopy) was very low: 365 cells/mm2 (MCE was noted previously at baseline).
We decided to switch from a fluid-ventilated design to an air-ventilated design (Figure 2), with goals of providing more oxygen to the cornea and minimizing suction. This allowed him to retain 20/30+2 BCVA for up to six hours of continuous wear without MCE. The patient was referred back to his cornea specialist for consideration of either a DSEK or a repeat full-thickness transplant OS.

Figure 2. Air-ventilated custom scleral device for the left eye (2010).
We refitted the right eye with a custom scleral device and had the patient wear the device overnight (off-label) with 1 drop of preservative-free Vigamox (Alcon) 0.5% in the device reservoir as prophylaxis to resurface the PED (Rosenthal et al, 2000; Lim et al, 2013; Ciralsky et al, 2015). We evaluated the patient every morning (including the weekend), with morning device removal, cleaning, and disinfection. We monitored re-epithelialization and stromal thickness with daily photo documentation, and we documented pertinent negatives, such as no keratic precipitates, no infiltrates, no hypopyon, and no anterior chamber (A/C) reaction. Figure 3 shows PED healing progression at days 1, 15, and 16 of treatment. BCVA in the right eye after healing of the PED improved from 20/200 with the bandage contact lens to 20/40+2 with the scleral device in place.
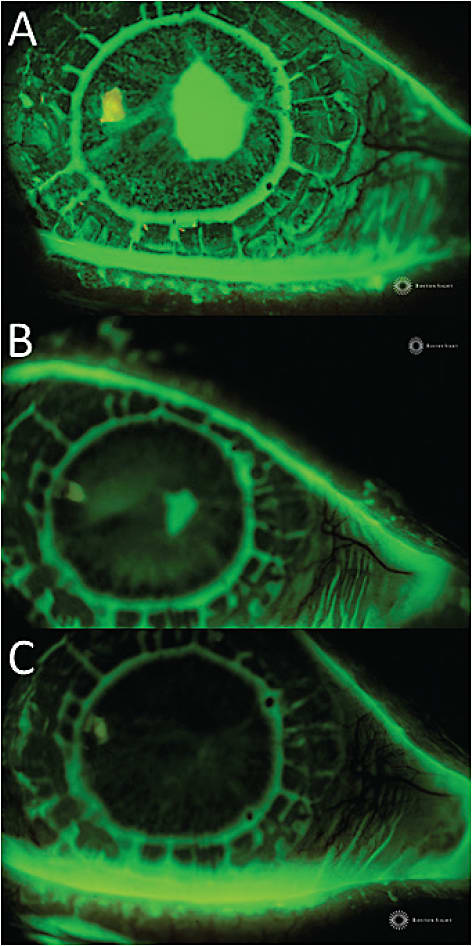
Figure 3. Persistent epithelial defect at A) day 1 (baseline), B) day 15, and C) day 16 after overnight wear with custom scleral device with daily clinical evaluation (including weekend days), removal, cleaning, disinfection, and reapplication with 1 drop of preservative-free Vigamox in the device reservoir.
The patient was refitted in 2010 with custom scleral devices with the following parameters:
OD: Sag height 2790µm @ 12mm chord, BC 8.0mm, –2.75D power, 18.5mm OAD, bitoric haptics, Boston XO2 material.
OS: Sag height 2650µm @ 12mm chord, BC 6.9mm, –5.75D power, 18.5mm OAD, two fenestrations, quad-toric haptics, Boston XO2 material.
The patient returned to his cornea specialist for continued care and medical management. We evaluated him again in May 2011 following a repeat transplant in the left eye. As with previous experiences, his ocular surface was slow to re-epithelialize after penetrating keratoplasty, and he had an 80% PED with stromal folds after his last evaluation with his cornea specialist one week prior to returning to us. Medications at the time included Pred Forte 1% and Vigamox q.i.d. OS, timolol 0.5% b.i.d. OD and OS, and Pred Forte 1% b.i.d. OD. A hydrogel bandage contact lens was in place status post (s/p) repeat penetrating keratoplasty.
At the time of evaluation in 2011, BCVA with a custom scleral device was 20/20 OD and 20/400, PH NI with the bandage lens in place OS. Mild conjunctival staining was noted after device removal in the right eye with associated tenderness (bitoric haptic design from 2010). In the left eye, there was no PED s/p bandage lens removal, but there were +1 to +2 stromal folds and bullae (Figure 4).

Figure 4. Repeat corneal graft with no persistent epithelial defect at the time of evaluation after bandage contact lens removal in the left eye (2011).
We refitted devices with the following parameters:
OD: Sag height 2400µm @ 12mm chord, BC 9.0mm, +3.50D power, 18.5mm OAD, quad-toric haptics, Boston XO2 material.
OS: Sag height 2250µm @ 12mm chord, BC 6.9mm, +1.25D power, 18.5mm OAD, quad-toric haptics, Boston XO2 material.
Best-corrected vision after refitting was 20/20 OD and 20/40 OS. His ocular surface, comfort, and BCVA remained stable for the next two years. The patient unfortunately passed away from systemic complications of GVHD in 2013, but before that time we were able to provide him with a viable option to alleviate severe dry eye symptoms associated with GVHD, support his ocular surface, and provide adequate best-corrected vision for nine years with the use of scleral prosthetic devices.
Case 2: Graft-Versus-Host Disease
In 2013, a 65-year-old Caucasian male had a history of GVHD secondary to bone marrow transplant (two autologous and two allogeneic in 2012) for acute myelogenous leukemia (diagnosed in 2012). The patient was referred to our clinic to address symptoms of severe photophobia, foreign body sensation, and poor BCVA. Previous treatment approaches included silicone punctal plugs (2012) and punctal cauterization (2013) in both superior/inferior puncta in both eyes. Topical ocular medications included Lotemax (B+L) 0.5% q.h.s. OD and OS, Kineret (Sobi) t.i.d. OD and OS, Refresh PM (Allergan) q.h.s. OD and OS, and Tears Naturale Free (Alcon) preservative-free artificial tears p.r.n. OD and OS. Systemic medications included acyclovir p.o. 400mg q.d., tacrolimus p.o. 1mg q.o.d., voriconazole p.o. 200mg q.d., and alpha-lipoic acid p.o. 800mg q.d. BCVA was 20/70, PH 20/40 OD and 20/50+2, PH 20/30 OS.
Evaluation showed superficial punctate keratitis (Oxford III OD and II OS) in both eyes. There was an elevated subepithelial opacification consistent with Salzmann’s nodular degeneration inferonasally with overlying band keratopathy in the right eye and a similar elevated opacification (possibly early Salzmann’s) with overlying band keratopathy in the left eye. He was pseudophakic in both eyes. BCVA with custom scleral devices improved to 20/30-2 OD and 20/40, PH 20/30 OS. The patient also reported a significant decrease in dry eye symptoms.
After one week of treatment and application/removal training, we dispensed devices to wear over the weekend with the following parameters:
OD: Sag height 2000µm @ 12mm chord, BC 8.4mm, –2.25D power, 19.0mm OAD, bitoric haptics, Boston Equalens II (B+L) material.
OS: Sag height 2000µm @ 12mm chord, BC 7.9mm, –4.25D power, 19.0mm OAD, bitoric haptics, Boston Equalens II material.
Evaluation the following Monday revealed an epithelial defect approximately 3mm x 2mm in the left eye. The patient reported the left eye being painful after device removal; hence there was a question as to whether there was trauma to the left eye upon removal (Figure 5). BCVA was 20/30- OD and 20/50+2 OS.

Figure 5. A) Epithelial defect after first weekend of solo application/removal in a GVHD patient. B) The epithelial defect as outlined after reapplication of the custom scleral device.
After three days of overnight wear with the scleral device with daily clinical evaluation, removal, cleaning, disinfection, and reapplication with 1 drop of preservative-free Vigamox in the device reservoir, the PED in the left eye healed (Figure 6). We reviewed the application/removal process with the patient to prevent further injury. Once adequately supporting the ocular surface in the left eye, BCVA was 20/30, PH NI OD and 20/40, PH 20/30 OS.

Figure 6. A) Epithelial defect at day 1 and B) after 3 days of overnight wear with a custom scleral device in a GVHD patient.
This case highlights that the ocular surface in severe forms of ocular surface disease can be very vulnerable and fragile, and even minor trauma during the application/removal process or even minor exposure overnight could lead to PEDs. Some cases may have complications from limbal stem cell deficiency, which would compromise healing. Close monitoring and follow-up clinical evaluation is necessary to ensure successful outcomes and avoid further complications when fitting these prosthetic devices and using them for therapeutic applications.
After one year, the ocular surface of this patient has remained stable. He still experiences relief from the severe dry eye symptoms associated with GVHD, and his BCVA has improved to 20/30, PH NI OD and 20/20-2 OS.
Case 3: Sjögren’s Syndrome/Superior Limbic Keratitis
In 2012, a 55-year-old Hispanic woman who has primary Sjögren’s syndrome was referred for PROSE treatment because of severe photophobia, foreign body sensation, pain, and redness in both eyes. Systemic medications at the time included acetaminophen with codeine p.o. (oral liquid) q.h.s. p.r.n., Evoxac (Roxane Laboratories) p.o. 30mg t.i.d., Lopressor (Novartis) p.o. 25mg q.d., vitamin E capsule, vitamin A capsule, fish oil 2 Tbs q.a.m. and 1 Tbs q.h.s. Topical medications included Kineret t.i.d. OD and OS, autologous serum tears three to seven times a day, FML (Allergan) 0.1% p.r.n. OD and OS, GenTeal (Alcon) gel q.h.s. OD and OS, TheraTears (Akorn), and Systane Plus (Alcon) p.r.n. OD and OS.
The patient was previously evaluated by another cornea specialist in 2011 who had diagnosed her with superior limbic keratitis (SLK) OD and OS. At the time of diagnosis, one consideration was conjunctival resection surgery, because it has been reported that conjunctival resection allows for conjunctival re-epithelialization adhering to the sclera without tissue laxity and with an improvement in symptomatology (Gris et al, 2010).
During the patient’s consultation and evaluation, we noted superficial punctate keratitis (Oxford II to III OD and I OS), signs of SLK OD more than OS with associated conjunctival staining along the superior limbal/conjunctival area, and reduced tear breakup time (3 to 4 seconds) (Figure 7). BCVA with spectacle correction was 20/40-2, PH 20/30-2 OD and 20/30-2, PH 20/25-3 OS.

Figure 7. A) Conjunctival and perilimbal injection and redundant conjunctival tissue associated with SLK, B) superior conjunctival staining, and C) corneal staining and reduced tear breakup time, right eye more than left eye, respectively.
We fitted the patient with devices with the following parameters:
OD: Sag height 2800µm @ 12mm chord, BC 7.9mm, –0.75D power, 19.5mm OAD, quad-toric haptics, Boston Equalens II material.
OS: Sag height 2800 µm @ 12mm chord, BC 7.9mm, –2.75D power, 19.5mm OAD, quad-toric haptics, Boston Equalens II material.
Best-corrected vision with these devices was 20/20 in both eyes, and the patient reported significant improvement in her symptoms. She reported no photophobia, pain, or foreign body sensation. It is also worth mentioning that with wear of these devices, the consideration of surgical conjunctival resection has been deferred.
Case 4: Stevens-Johnson Syndrome
A 29-year-old Hispanic female developed SJS secondary to a reaction to Lamictal in 2005. She had bilateral corneal perforation and underwent subsequent penetrating keratoplasty in 2005, cataract extraction/posterior capsule intraocular lens (PCIOL) with synechialysis in the right eye and cataract extraction/PCIOL in the left eye (February 2009), electro epilation (May 2009), and repeat penetrating keratoplasty (four total) due to failed grafts in the right eye. She was referred to our clinic in March 2013 for treatment of a chronic PED after the most recent repeat penetrating keratoplasty in the right eye (August 2012). The defect was recalcitrant to previous treatments prior to consultation, including extended wear of an 18mm bandage contact lens, tape tarsorrhaphy, and Prokera (twice). The left eye had no PED and the graft was compact, but the patient reported severe pain and photophobia.
Systemic medications at the time of consultation included Cellcept (Genentech) p.o. 1g b.i.d. and doxycycline p.o. 200mg q.d. Topical ocular medications included Vigamox 0.5% q.i.d. OD, erythromycin 0.5% ointment q.i.d. OD, Pred Forte 1% b.i.d. OS, Restasis 0.05% b.i.d. OS, and preserved artificial tears q30min OD and OS. Past medical history was also relevant for depression and bipolar disorder.
BCVA in the right eye was hand motion, and uncorrected visual acuity in the left eye was 20/200; neither eye improved with pinhole. Evaluation revealed lid margin keratinization, distichiasis, several small keloids in right upper lid, and punctal stenosis in both eyes. There was corneal conjunctivalization, no infiltrates, no hypopyon, but a central epithelial defect (approximately 3.5mm x 3.5mm) (Figure 8) with 50% to 60% stromal thinning and profound central haze/failing transplant in the right eye. Additionally, there was corectopia in the right eye.

Figure 8. Persistent epithelial defect approximately 3.5mm x 3.5mm in size after a fourth penetrating keratoplasty in a Stevens-Johnson syndrome patient.
The left eye exhibited neovascularization superiorly and inferiorly, scarring superiorly and centrally with 10% stromal thinning, but no epithelial defect in the left eye (Figure 9).

Figure 9. A) Right cornea and B) left cornea after penetrating keratoplasty (fourth OD and first OS) secondary to corneal perforation in a Stevens-Johnson syndrome patient with custom scleral devices in place.
We fitted the patient with custom scleral devices in the following parameters:
OD: Sag height 2800µm @ 12mm chord, BC 7.9mm, 1.50D power, 18.0mm OAD, quad-toric haptics, Boston Equalens II material.
OS: Sag height 2600µm @ 12mm chord, BC 7.9mm, –0.50D power, 18.0mm OAD, quad-toric haptics, Boston Equalens II material.
Best-corrected vision with these devices improved from hand motion to 20/400, PH NI OD and to 20/50+2, PH NI OS. For the right eye, we started overnight device wear and evaluated the patient every morning (including the weekend), with morning removal, cleaning, and disinfection. We monitored re-epithelialization progression and stromal thickness with daily photo documentation, and we documented pertinent negatives, such as no keratic precipitates, no infiltrates, no hypopyon, and no A/C reaction. Figure 10 shows PED re-epithelialization at days 1, 7, and 20 of treatment.

Figure 10. Chronic persistent epithelial defect recalcitrant to conventional therapy in a Stevens-Johnson syndrome patient that healed with a scleral device/PROSE treatment: A) Baseline day 1, B) day 7, and C) day 20.
In this case of SJS/limbal stem deficiency, specialists had been struggling for eight months to heal this PED in a fragile cornea that had undergone its fourth penetrating keratoplasty. The defect resurfaced after 20 days with PROSE treatment. This case, like the others presented here, shows how achieving prosthetic replacement of the ocular surface with scleral devices provides the ocular surface with an adequate environment to support healing. Also, in SJS cases in particular, they help protect the cornea from the environment, especially from the transformed eyelids and lid margins.
After one-and-a-half years of treatment, the ocular surface in the right eye remains intact, and there has been no further breakdown of the epithelial surface. We have continued with PROSE treatment in the right eye despite poor BCVA secondary to the failed graft, with the goal of supporting the ocular surface; due to multiple surgical procedures in the right eye, the patient is hypoesthetic with a neurotrophic component. Treatment in the left eye has allowed the patient to be pain-free and photophobia-free with improved BCVA, allowing for an overall improvement in quality of life.
Scleral Lens Management for Severe Ocular Surface Disease
These cases illustrate the therapeutic and visual benefits that custom scleral devices can offer to patients who have severe ocular surface disease. Managing such fragile ocular surfaces with scleral lenses involves the following special considerations.
Vault/Clearance The classic definition of a well-fit or customized scleral device is that it completely vaults the corneal surface, including the limbus. There can be instances when using scleral lenses for an irregular cornea case, such as keratoconus, in which some incidental touch or bearing occurs because of unanticipated amounts of device settling. Usually, this is not a problem if the bearing is evenly distributed and the cornea is healthy, although the overall goal is to prevent the risk of potential mechanical neovascularization.
This scenario should be avoided when using custom scleral devices for therapeutic indications in the context and treatment of severe ocular surface disease. These eyes have compromised ocular surfaces, and many of these cases will have some degree of limbal stem cell deficiency. Complications may include keratitis with increased staining, neovascularization, and peripheral corneal edema, with associated symptoms of patient discomfort, decreased tolerance, and reduced BCVA.
How much central vault is necessary to successfully fit this subset of patients? Sonsino and Mathe (2013) reported that a specific central vault was not important for patients who were fit with scleral lenses to manage dry eye. The mean vault to successfully fit such patients was 380 microns, with a standard deviation of 110 microns. Scleral lens designs (mini-scleral and full scleral lenses) will often fully vault the limbus if a patient has an average horizontal visible iris diameter (HVID) of 11.8mm (Caroline et al, 2002). If the cornea has an HVID larger than 12mm, then the likelihood increases that limbal touch may occur. Increasing the lens diameter will often improve the vault over the limbus for larger-than-average corneas.
Reservoir There is little to no tear exchange with a well-fit scleral lens (Morrison, 2012), which means that the initial reservoir of solution that is instilled prior to application will have hours of contact with the ocular surface. For this reason, it is critical that patients who have ocular surface disease use inert/preservative-free solutions to eliminate the risk of toxicity or hypersensitivity reactions. Prescribing off-label use of nonpreserved saline solutions can prevent an avoidable complication. In addition, antibiotics used for prophylaxis in the treatment of PEDs should also be preservative-free.
Reservoir Debris Patients who have severe ocular surface disease often produce copious amounts of debris and mucus that can get trapped in the reservoir. Reservoir debris can cause significant visual disturbance and loss of acuity. The debris is more likely to get pumped behind the lens if there are gaps resulting from a spherical-back-surface scleral lens on a non-spherical sclera. Fitting scleral lenses with back-surface toricity or quadrant-specific curves that match the astigmatic shape of the sclera should help to reduce reservoir debris. Using a viscous, preservative-free artificial tear in the reservoir in addition to saline may also prevent debris intrusion in the reservoir.
Application Applying a saline-filled scleral lens successfully can be challenging for any patient. Patients who have other systemic issues or disabilities may have increased difficulty. Consider the following techniques if standard application training is unsuccessful. Involve caretakers during application training, as they may need to help the patient at home during the initial learning curve. Stands are commercially available that hold application plungers with scleral lenses positioned on top, which allows patients to use both hands to help maximize lid separation. Filling the scleral lens with off-label use of carboxymethylcellulose will help reduce spillage if a patient is unsteady and having trouble achieving bubble-free application.
Co-Management Patients who have severe ocular surface disease typically have multiple physicians who manage various elements of their primary disease. Often, these patients will be concurrently under the care of a cornea specialist. Make sure to properly communicate with the other individuals who are involved in their care. Some of these patients may be able to significantly decrease the number and amount of over-the-counter and prescription ophthalmic medications that they take once they are successfully wearing scleral lenses on a daily wear basis. If applicable, any anticipated changes to their ocular topical medications should be discussed with their cornea specialist.
Patients and Referrals Unfortunately, it is not widely known that scleral lenses can help patients who have severe ocular surface disease. Introduce yourself to local specialists, including cornea specialists, to inform them that you fit these devices. Supportive materials, such as peer-reviewed papers or case reports, may help them understand how scleral lenses can benefit this subcategory of their patient population.
Make sure to add scleral lenses to your web page to attract patients—patients search the Internet for scleral lens providers when they find out that scleral lenses may improve their situation. Testimonials from previous cases can go a long way toward gaining initial trust concerning your skills and success.
Conclusion
It’s unfortunate that there are patients who have severe ocular surface disease and could significantly benefit from scleral lenses, yet they have not been properly referred for a scleral lens evaluation. Fitting scleral lenses to manage ocular surface disease can be more challenging and time consuming compared to fitting them for patients who have irregular corneas. More frequent follow-up visits are necessary to continually monitor for the development of any secondary complications, such as corneal edema and neovascularization; in cases of chronic PEDs, it is paramount to monitor for and rule out progressive stromal thinning that could lead to corneal melting or rupture.
Aside from frequent monitoring and follow-up care, it is crucial that you have a good relationship and communication with a patient’s referring corneal specialist and/or oncologist/rheumatologist to ensure effective co-management of these challenging cases. Accepting these challenges will allow you to significantly improve the quality of life for patients who suffer with decreased ocular comfort and vision associated with complex corneal disease. CLS
The authors acknowledge Lynette Johns, OD, FAAO, for her assistance in preparing this article.
For references, please visit www.clspectrum.com/references and click on document #239.

| Dr. Carrasquillo is director of Clinical Care at the Boston Foundation for Sight, adjunct clinical faculty at the New England College of Optometry, adjunct clinical faculty at the School of Optometry, MCPHS University, and an Advisory Board member of the Gas Permeable Lens Institute. She has also authored various publications in the therapeutic use of scleral devices and prosthetic replacement of the ocular surface system (PROSE) treatment. |

| Dr. DeNaeyer is the clinical director for Arena Eye Surgeons in Columbus, Ohio and a consultant to Visionary Optics, Alcon, B+L, and Aciont. He is also a shareholder in Precision Ocular Metrology LLC. You can contact him at gdenaeyer@arenaeyesurgeons.com. |



