There is no argument today that the number one problem in managing our scleral lens patients is surface non-wetting and subsequent deposit formation. In part, this problem stems from the core formulation of the fluorosilicone-acrylate (FSA) materials of which GP lenses are made.
These FSA materials contain three primary ingredients: 1) silicone and fluorine for oxygen permeability; 2) methacrylic acid and HEMA for material wetting properties; and 3) methylmethacrylate for mechanical and optical stability. These liquid monomers are mixed together with cross-linking agents and are then polymerized (hardened) to form the bulk material out of which GP lenses are fabricated (Figure 1).
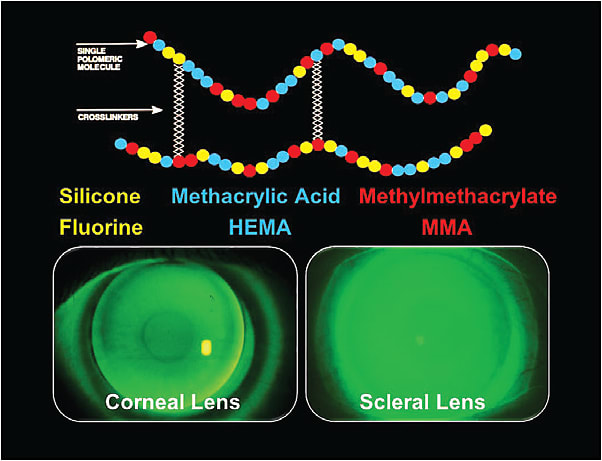
GP Surface Modification
In the formulation of high-Dk (100+) materials for scleral lenses, the proportions of the monomers have been stretched to their physical limits to the point that any attempt to further increase oxygen permeability (increased silicone and fluorine content) will further compromise the material wetting, and any increase in wetting properties (increased methacrylic acid and HEMA) will lower the material Dk. Therefore, the only avenue left to enhance the surface properties of these lenses is through molecular surface modification or surface coatings.
At the 2015 Global Specialty Lens Symposium (GSLS), we presented a poster in which we discussed the use of a new 90% water polyethylene glycol (PEG) polymer that can be bonded to the surfaces of both rigid and soft contact lenses. This coating encapsulates the core contact lens with a a mucin-like hydrophilic shell to improve surface wettability and deposit resistance (Figure 2). PEG polymers have a long history of use in a wide range of medical applications, such as ocular medications, wound care products, and oral suspensions.

In September 2016, this lens surface coating received FDA approval for use with select GP lens materials. The coating was officially launched by a number of U.S. contact lens laboratories at the GSLS in January 2017.
Improving Lens Wettability
Our patient is a 42-year-old male with a history of post-laser-assisted in situ keratomileusis ectasia and a penetrating keratoplasty to his right eye. The patient wore his scleral lenses for 12 hours a day; however, he needed to remove them every four hours to manage problems with surface deposition and tear film fogging.
We placed him into the surface-treated lenses, and he immediately reported improved lens comfort and wear time of 16 hours per day. He experienced complete resolution of his surface deposits and tear film fogging. Figure 3 shows the anterior tear film breakup of the patient’s lenses prior to and following the surface treatment.
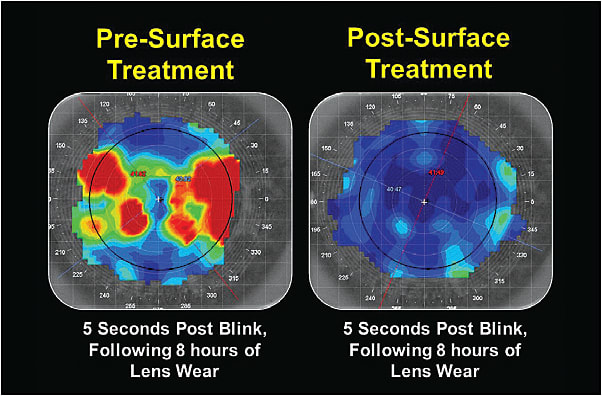
The major unanswered question is: How long will the surface coating last? Only time can provide clarity to that question. CLS





