Scleral lenses have gained popularity in recent times, with Bergmanson, Barnett et al declaring in 2016 that scleral lenses have “come of age,” describing them as the first choice of lens for the irregular cornea.
What Are Scleral Lenses?
The Scleral Lens Education Society (SLS) defines scleral lenses as those that rest entirely on the sclera, no matter the size of the lens (SLS, 2013). These are further subdivided into mini-scleral or large scleral lenses; the former relates to lenses up to 6mm larger than the horizontal visible iris diameter (HVID), and the latter relates to lenses more than 6mm larger than the HVID (SLS, 2013).
Scleral lenses are often used to manage severe ocular surface disease, making the need to understand their impact on the ocular surface even more pertinent. The size of the lens, completely vaulting the cornea and the limbus, means that the epithelium is protected from the shear forces of the eyelids during the blink, allowing the epithelium to recover without the constant interaction with the eyelids. The vault over the cornea results in a tear fluid reservoir that can improve the optical quality of the ocular surface (Tan et al, 1995; de Luis Eguileor et al, 2016). This can also have physiological benefits.
In a review of 530 cases of scleral lens wear, 53% were comprised of keratoconus, keratoglobus, and pellucid marginal degeneration (Pullum and Buckley, 1997). They have also been used for tear retention in severe dry eye disease in cases of exposure keratitis (Pullum and Buckley, 1997; Weber et al, 2016), neurotrophic keratopathy (Schornack et al, 2014), and in Graves’ ophthalmopathy (Harthan, 2014). Scleral lenses have also been used as a method of drug delivery (Laballe et al, 2016) and after penetrating keratoplasty (Barnett et al, 2016).
How Are Scleral Lenses Fitted?
Sclerals are designed to clear the cornea and to rest on the conjunctiva. Some designs aim to maximize oxygen transmission by reducing the lens diameter or by reducing the amount of corneal clearance.
In a recent survey of prescription and management practices, average scleral lens wear time was 11.8 hours, with most clinicians recommending lens removal at midday. The majority of the clinicians surveyed recommended nonpreserved solutions for the liquid reservoir and peroxide-based systems for the disinfection process (Harthan et al, 2017).
Scleral lenses go against the soft contact lens fitting philosophy; achieving a good tear film exchange to minimize the risk of adverse events is less of an option. Walker et al (2016) discuss “lens seal-off,” which they describe as a strong suction between the lens and the ocular surface that makes it hard to remove the lens and minimizes the exchange of tears. The increased risk of inflammation with the reduced tear exchange leads many practitioners to recommend midday lens removal (Harthan et al, 2017).
Complications with Scleral Lens Wear
Discontinuation The discontinuation rate with scleral lenses has been reported to range between 19% and 29% (Pullum and Buckley, 1997), with the most common reason cited in one paper being the trouble with application and removal (Asena and Altınörs, 2016).
Microbial Keratitis and Epithelial Abrasions Several studies have monitored the complications associated with scleral lens wear. Nixon and colleagues (2017) present a series of case studies documenting corneal epithelial bullae and epithelial erosions (Figure 1). Of the 115 patients followed by Schornack et al (2014), three developed complications relating to scleral lens wear: one corneal abrasion during the application process, one case of microbial keratitis, and one limbal epithelial defect. Rosenthal and Croteau (2005) have also reported an incidence of microbial keratitis of 1.6%. While low, this suggests the need for careful vigilance of patients fitted with scleral lenses, as these are people whose ocular surface is already compromised.
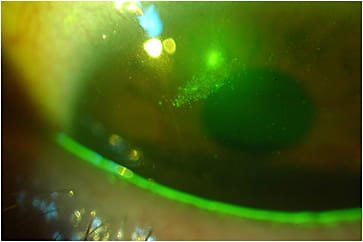
Ocular Surface Inflammation Little is known about whether scleral lens wear has any effect on ocular surface inflammation. Carracedo, Blanco, and colleagues (2016) reported an increase in the collagen degrading enzyme matrix metalloproteinase-9 (MMP-9) in the tear film following eight hours of scleral lens wear in a group of keratoconus patients, attributing this to the stagnation of the tear film. Similarly, Weber and colleagues (2017) found an increased expression of Human Leukocyte Antigen–Antigen D Related (HLA-DR), a proinflammatory marker, in people who have Sjögren’s syndrome fitted with scleral lenses. No impact on ocular surface temperature has been recorded with scleral lens wear (Carracedo, Wang et al, 2016).
Corneal Hypoxia The impact of scleral lens wear on corneal hypoxia is debated in the literature. Jaynes et al (2015) calculated the theoretical oxygen tensions of the tear layer between the scleral lens and the corneal surface. They found that only the best case scenario of a 300µm lens combined with a 50µm-thick tear film and a 140-Dk scleral lens allowed adequate oxygen to the ocular surface.
They recommended fitting lenses with high Dk, low corneal vault, and minimal acceptable scleral GP thickness to minimize hypoxia, but conceded that this is not always realistic in the clinical setting due to the unique characteristics of the diseased corneas usually fitted with these lenses. Also, the theoretical calculations do not take into account the potential tear exchange between the post-lens reservoir and tears peripheral to the lens.
Corneal Nerve Structure and Function Wang et al (2015) explored the impact of scleral lens wear on corneal nerve structure and function in eyes with distorted corneas or ocular surface disease. In the study, subbasal nerve density and tortuosity remained unchanged following long-term scleral lens wear, while corneal sensation increased and tear production decreased in those who have distorted corneas.
Landing Zone Compression The fluid reservoir has been shown to decrease by 50% over a two-hour wear period as the lens settles onto the ocular surface (Nau and Schornack, 2017). It has been hypothesized that the reason for this reduced fluid reservoir, and the resultant reduced apical clearance, may be compression in the landing zone of the lens (Alonso-Caneiro et al, 2016). The authors explored this hypothesis by assessing the effect of mini-scleral contact lenses on the morphology of the scleral lens landing zone.
They found significant thinning in all quadrants, with the majority of the compression occurring in the conjunctival and episcleral region, with the sclera minimally affected and the greatest compression occurring superiorly (Alonso-Caneiro et al, 2016). Only the inferior region returned to baseline thickness during the three-hour follow-up period. The authors attribute this to the strength of the scleral network and the pressure of the eyelid, respectively. They did not, however, find a significant correlation between apical clearance and the amount of landing zone compression, suggesting another cause of reduced fluid reservoir with lens wear.
Intraocular Pressure (IOP) It has been hypothesized that scleral lenses elevate IOP due to an increase in corneal bearing and conjunctival and scleral pressure as a result of post-lens fluid forces being augmented by eyelid tension and eye movements (McMonnies, 2016), an argument that has been countered (Vincent et al, 2017; Pearson, 2017). In a review by Vincent et al (2017), the change in IOP was found to be less than 1.5 mmHg when worn for less than eight hours. In fact, they have shown a similar diurnal variation in IOP either with or without lens wear, whether the lenses were worn for three or eight hours. Similarly, Nau and colleagues (2016) showed that two hours of lens wear did not significantly affect IOP.
This suggests that the tissue compression near the scleral spur does not significantly impact IOP. However, well-designed studies are needed to address this question once and for all. In the meantime, use caution, particularly when fitting patients who have ocular hypertension or filtration devices near the limbus (Vincent et al, 2017).
Midday Fogging One of the most commonly reported events associated with scleral wear is midday fogging (Bergmanson, Walker et al, 2016). This results in temporary disturbance to vision, with vision returning to normal when the lens is removed and a new reservoir added. “Particulate matter” accumulates in the tear fluid reservoir, contributing to this effect (Walker et al, 2016). This matter can accumulate very early on in the lens wear period or after a few hours (Walker et al, 2016). Very little is known about what this actually is or what significance it has. Walker et al (2016) recommend the use of high-viscosity, ion-containing, preservative-free artificial tears for the fluid reservoir to minimize this occurrence.
Conclusion
A greater understanding of the impact of scleral lenses on corneal physiology is being developed. This is important to ensure adequate monitoring of patients fitted with sclerals. In the case of silicone hydrogel soft contact lenses, the industry was propelled forward through the need to minimize hypoxia and mechanical events such as superior epithelial arcuate lesions. By understanding the complications that result from scleral lens wear, improvements can be made in their design, and an evidence-based consensus can be developed as to the best ways to fit scleral lenses. CLS
For references, please visit www.clspectrum.com/references and click on document #260.





