Julia, an 11-year-old female, is consulting you on referral for orthokeratology (OK) because her parents are concerned about her myopia progression. She is now wearing –2.00D glasses and was first corrected at 9 years old (–0.75D).
Julia presents several risk factors: genetic background (Dad: Caucasian, –3.00D; Mom: Asian –8.00D), reduced time spent outdoors, and computer work and playing on tablets for two hours/day). Her vision examination reveals a cycloplegic refraction of OD –2.50 –0.50 x 180 (20/20) and OS –2.25 –0.75 x 165 (20/20). She is esophoric at a distance and exophoric at a close range, with a normal accommodative lag. Is she a good candidate to be fitted with OK lenses? The following eight elements can help us answer this question.
ELEMENT #1: CLINICAL ASSESSMENT TO ESTABLISH A TARGET FOR MYOPIA CONTROL
Before concluding on any one strategy to achieve effective myopia control for Julia, it is important to carefully evaluate all of the assessments necessary to perform comprehensive myopia control:
- Targeted case history (background, documented evolution, genetics, environmental conditions, risk factors)
- Binocular vision assessment with best-corrected visual acuity (BCVA) far and near (phoria, accommodative convergence/accommodation [AC/A] ratio, lag of accommodation)
- Topography mapping (axial and elevation maps, corneal eccentricity)
- Axial length (AL) measurement (biometry or A Scan)
- Pupil size evaluation (pupilometer or from biometry)
- High-order aberration (HOA) evaluation (aberrometry)
- Ocular health assessment (anterior and posterior segments)
- Cycloplegic electronic auto-refraction
Most practitioners may not have access to biometry or aberrometry; however, these instruments are becoming more and more essential and are no longer restricted to the research arena. It is important to monitor HOAs from baseline to customize the myopia control strategy and evaluate its outcome. The most important aspect to consider here is the level of spherical aberration (SA). Valuable myopia control strategy (MCS) devices would increase/induce positive SA.1 Second, whereas high HOA levels, which are associated with symptoms of haloes and glare, may be tolerated at a younger age, they can become annoying for older patients while driving or doing a lot of computer work.
AL measurement is the only objective method that provides an indication of myopia progression over the years. It is also considered a key marker to establish the potential of developing retinal disease at an older age. In fact, the cumulative risk to be visually impaired, less than 20/60 VA at 75 years old, increased from 3.8% in eyes with an AL of less than 26mm to 25% in eyes with an AL of 26mm or more.2
ELEMENT #2: EVALUATE THE POTENTIAL EVOLUTION
There is no truly safe level of myopia related to the risk of developing pathology, which increases by 17% for every diopter of progression.3 However, an MCS may not be required for all myopes at the moment of their consultation. Someone becoming myopic at 17 years old, with no genetic background and limited risk factors, will not be managed the same way as someone who is 8 years old, already at –3.75D, and with both parents highly myopic.
Evaluating the potential evolution is a crucial step in establishing the right strategy for our patients. We developed the following formula, which helps to determine whether it is necessary to initiate an MCS or to simply observe the condition.
- Based on AL, treatment is needed immediately if:


Where - ALc = Critical AL (26mm by default)
- ALb = AL at baseline
- Age St = Estimated age for stabilization (18 years old by default)
- Age = Age of the patient at time of MCS consideration
- Prog = Progression—estimated or measured/year (average). Estimated AL Prog: Caucasian 0.2mm/year, Asian 0.3mm/year by default. As a reference, 0.1mm increase in AL represents 0.25D of myopia progression.
- Based on myopia, treatment is needed immediately if:


Where - Mc = Critical myopia (–6.00D by default)
- Mb = Myopia at baseline
Julia’s baseline myopia, as evaluated recently, is –2.75D and –2.50D (spherical equivalent), up from –0.75D two years ago. The average progression is then –0.87D/year. Her AL is 24.8mm, and its progression is unknown. The default value is used in this equation:

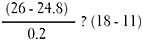
Solution: 6 < 7

Solution: 4.37 < 7
Based on the AL and myopia, Julia is considered at high risk for developing pathological myopia. An MCS should be suggested to the parents and implemented immediately.
Another way to address this question is to rationalize the potential evolution of a candidate. We all know that myopia never evolves in a linear progression and that many variations can occur over the years; however, the estimated values given earlier may be used. For example, consider a child who has a heavy genetic background and is evolving by –1.00D per year on average and 0.3mm per year AL; if this child is 8 to 14 years old, it is likely that his or her myopia will become at risk for future pathology development. Optical means will allow us to reach 50% effectiveness, which keeps myopia evolution at 0.50D per year and AL at 0.1mm to 0.2mm per year. If the child is already at –4.00D at 7 years old, you know that this strategy will not alleviate the potential negative outcome. You must consider an adjunct therapy, such as low-concentration atropine, in addition to optical interventions.
ELEMENT #3: WHICH IS THE BEST STRATEGY?
Based on reviews published about myopia control, we can expect the following average rates of myopia control with several strategies:
Before assuming that atropine offers optimum control, it is very important to look at studies conducted with each strategy and analyze the clinical population studied and the differences in the design of the lenses used. Once completed, key points can be identified:
- Genetic background plays a significant role; Asians cannot be managed in the same way as Caucasians.7
- Age of myopia onset drives evolution; we should be more aggressive when myopia occurs before 10 years of age, especially in female patients.8
- OK does not seem to work as effectively on lower myopia, with the exception of some customized designs.9
- For multifocal lenses, an add power of +3.00D and higher is optimal to control myopia.10 The add power should be balanced in light of distance blur. Distance-center and concentric ring designs appear to be slightly more successful compared to other designs.11
In addition to the literature, we looked at our own set of data. The Université de Montréal clinic database includes 600 files of children seen for myopia control. For OK lenses:
- Larger treatment zones are associated with less favorable outcomes. In certain cases, some commercial OK lenses can lead to myopia progression rather than myopia control. This means that OK, with commercial non-customizable treatment zones, is not a suitable option for every patient.
- Hydraulic forces are enhanced if the lens system is sealed off more compared to OK used for myopia correction. Peripheral curves should be modified accordingly (e.g., the landing zone angle or equivalent should be prescribed with one angle increase).
- Larger-diameter lenses (90% to 95% of the corneal diameter) are associated with better outcomes.
- Centration of the lens is crucial, and adjusting peripheral curves becomes essential. Using an elevation map, any difference between the two primary meridians, superior to 30 microns, drives the need to use toric peripheral curves (or dual-axis designs). Otherwise, hydraulic forces are not applied symmetrically, and lenses tend to decenter.
- A second reservoir zone, shallower in height, helps to enhance lens evaluation, leads to a more favorable outcome, and helps stabilize the lens.
To summarize our findings on OK lenses, it seems clear that most commercial lenses that are designed for myopia correction are not the best tools for myopia control without full customization. In some cases, the misuse of these lenses can even lead to myopia progression rather than myopia control.
This is why, as a general recommendation, practitioners should select lenses that are fully customizable or design their lenses with fitting software. If this is not possible, Figure 1 illustrates when OK represents the best approach for myopia control.

The remaining elements will focus on the use of OK lenses.
ELEMENT #4: HOW DO WE USE OK ON LOW MYOPES?
We usually deem that the amount of plus power generated with a regular commercial OK lens is slightly greater (25%) than the amount of myopia correction considering that the Jessen compression factor formula underestimates the effective power change on the cornea.12
This 1 to 1.25 ratio explains why OK does not generate sufficient addition to significantly modify peripheral refraction in low myopic children.
Analysis of our database indicates that myopia is best controlled when OK lenses generate 4.00D to 5.00D of plus power. We can see some control if the add power is as low as 2.50D, but the effectiveness of the control is then reduced. This may partially explain why some articles reported 50% efficacy with OK while others demonstrated 80% control of myopia or AL evolution.
The power generated by the lens is important, but most of it should also lie within the pupil area. If most of the plus power is generated outside of the pupil, OK lenses will act exactly like a spherical monofocal lens, and myopia will increase. This is why it is very important to be able to customize all aspects of OK lenses.
Customization will also include posterior asphericity. If we want to use OK lenses on low myopes and establish a higher ratio than 1:1.25 versus myopic correction, then higher back asphericity is needed. In doing so, the diameter of the treatment zone will be reduced. If the diameter is too limited, distance blur may be reported, and visual acuity will be negatively affected.
Customization is also possible by modifying the number, the radius, and the width of intermediate and peripheral curves in a quadrant-specific approach. Some manufacturers in Europe are testing dual-focus system lenses that are similar to soft multifocals based on alternating ring designs (called mOK) to enhance outcomes in low myopes.
In summary, for any myopic patient who has a refractive error of less than 3.50D, regular OK lenses designed for myopia correction (and not control) will not be successful, and their design should be customized to generate more plus power or more central rings. If practitioners do not have access to such customized lenses, or if they are not able to design them, then soft multifocal lenses would be preferred for low myopia.
ELEMENT #5: PARTIAL OK IS OK
The amount of myopia for which we can compensate is directly linked with corneal eccentricity. It is recognized that higher myopia correction with OK is more effective most likely because of a higher plus power generated in the periphery.13 Mountford’s rule14 establishes that the maximum for which we can compensate is equal to the highest corneal eccentricity/2 + 1.00D. To correct more than this, the compression factor must be increased. In such a case, the treatment zone will be severely reduced; consequently, blurred vision will occur at a distance, and HOAs will increase, generating more haloes. More importantly, higher myopia correction is positively linked to higher significant corneal staining,15 which may also increase the risk of developing infections and corneal ulcers.
To remain on the safe side, and despite the fact that software can allow us to design OK lenses for myopia up to 6.00D to 7.00D, our clinic adopted the policy of never attempting to compensate for more than 4.50D of myopia, which is sufficient to generate enough plus power to obtain full myopia control. Any refractive error that remains is compensated for with a pair of regular glasses; this has no impact on the MCS, and the cornea remains reshaped throughout the entire day. This safe approach allows us to avoid significant adverse events.
This strategy is called partial reduction OK and was found to be as effective as full correction of myopia.16 In fact, those patients tend to present a higher rate of myopia control (80% reduction) compared to those who have lower myopia. This emphasizes the importance of differentiating between myopia correction and myopia control.
ELEMENT #6: ADDRESS ASTIGMATISM
In the OK world, corneal astigmatism plays a significant role in lens fitting and in the outcome generated by lens wear.17 It also represents a real challenge for the design of the ultimate lens. We have to remember that OK lenses modify corneal shape by applying negative and positive hydraulic forces to the tissue. In the presence of a spherical lens on a toric corneal surface, it is difficult to generate symmetrical forces; consequently, treatment is suboptimal, and the lens itself, following the path of the least resistance, will decenter.
Any corneal astigmatism greater than 1.00D requires the use of a toric-back-surface optic zone, and if the toricity extends from limbus-to-limbus, peripheries should also be designed differently in principal meridians.17 Once again, customization is the key, and practitioners may need to consult manufacturers to modify current designs as needed.
Once designed with a toric-back-surface optic zone, the fluorescein pattern will be seen as oval-shaped, and accordingly, the tangential map on topography will duplicate it. It is important to maintain good lens centration to make sure that the correction is efficient.
ELEMENT #7: WHAT IF MYOPIA EVOLVES?
The MCS aims to limit the progression of myopia and, more importantly, AL over time. It is practically impossible to achieve 100% control given the natural growth of children. Consequently, it is very possible to observe myopia progression occurring despite an optimal OK fit. What can we do then?
Before concluding that myopia has evolved, we must take into account the following considerations:
- Timing of the Follow-Up Consultation Children seen late in the afternoon may show some myopia as a consequence of corneal restoration over time. Depending on the symptoms and visual needs, low-myopic-power glasses may be prescribed to be worn when vision becomes blurry at a distance at the end of the day.
- Type of OK/MCS Partial OK is obviously associated with residual myopia, which can also evolve during the day as the cornea tries to restore its natural shape.
- Baseline AL in OK In patients undergoing OK treatment, a new baseline AL should be taken one month after initiating the treatment. Given the flattening surrounded by a steepened zone in the center of the cornea, the AL can be artificially increased by the biometer in some cases. The one-month AL measurement will become the new baseline for the follow up. If available, other parameters may be considered (crystalline lens thickness, anterior chamber volume, etc.) to establish where the variation comes from.
- Cycloplegic versus Non-Cycloplegic Refraction Some young patients can display accommodative spasm, clinically associated with pseudo-myopia, despite a successful OK treatment.
The true test to perform is to over-refract when lenses are worn. In the presence of real myopia evolution, over-refraction will require more minus power to reach 20/20 at a distance. If myopia evolves, or AL increases, we must determine whether this evolution is within the expected limits or whether it is excessive compared to our predictions. For example, consider a patient known to evolve 1.00D per year before implementing the MCS; with customized OK lenses, the expectation is to limit this progression by 50%, which results in 0.50D myopia increase/year or 0.1 to 0.2 mm/year for AL, which would be considered normal. If the myopia evolution exceeds this amount, the MCS should be revisited. Several options may include:
- Modify the OK Lenses Naturally, this is the first step that comes to mind, but it is most likely the last one that should be considered. To compensate for higher myopia would require increasing the positive hydraulic pressure in the central cornea by flattening the base curve(s). This can be done only if there is sufficient space to do so, according to Mounford’s formula. Otherwise, this may require increasing the mechanical pressure and, consequently, the risk of corneal erosion.
- Adopt a Partial OK Approach Any residual myopia may be corrected with regular glasses during the day, knowing that the cornea remains molded from the overnight OK and that peripheral refraction is still influenced by the plus-power ring generated by lens wear.
- Consider Adjunct Therapy If the evolution exceeds expectations, it may be a good idea to consider an adjunct therapy. A single report mentioned the combined intervention of OK lenses and low-dose atropine (0.01%).18 It seems that both strategies add up and provide a better outcome. For patients who have a limited pupil diameter, combined use of atropine may also help to enhance OK results, given that it dilates the pupil 1mm to 1.5mm when used.
- Consider Switching Strategies It was suggested to switch to a different strategy every two to three years, based on the fact that most clinical effects on myopia or AL progression occur during the first year of treatment.19 If this occurs, switching over to soft multifocal lenses or atropine as a stand-alone therapy may make sense.
ELEMENT #8: WHAT ABOUT BINOCULAR VISION?
It is well documented that lag of accommodation, esophoria for nearsightedness, and an abnormal AC/A ratio are all associated with myopia progression.20 The MCS should address any binocular vision abnormalities seen at baseline through vision therapy, prismatic glasses, or other means.
Once started, any MCS should also be reevaluated in light of its potential effect on binocular vision. Gifford (2017) looked at the clinical effect of OK on several aspects of the binocular function.21 As expected, she confirmed an exophoric shift, improved divergence, reduced accommodative lag, and no significant clinical effect on convergence when OK is used.
DEFINING THE LONG-TERM STRATEGY
OK is one of the most effective strategies to control myopia and slow AL growth. Before selecting it as the method of choice, practitioners should define and customize the long-term strategy for each patient. Figure 2 shows an algorithm that may help.

The MCS should be adapted to each patient with the big picture in mind. There is no unique recipe or magic bullet that will fit every child or myopic individual.
The same is true for OK. Most standard commercial OK lenses were developed for myopia correction. When fitting OK for myopia control, practitioners need to adjust the parameters and customize the design of these lenses to optimize the outcome. Practitioners should become familiar with these differences before considering OK as their MCS of choice. CLS
REFERENCES
- Cheng X, Xu J, Chehab K, Exford J, Brennan N. Soft Contact Lenses with Positive Spherical Aberration for Myopia Control. Optom Vis Sci. 2016 Apr;93:353-366.
- Tideman JW, Snabel MC, Tedja MS, et al. Association of Axial Length With Risk of Uncorrectable Visual Impairment for Europeans With Myopia. JAMA Ophthalmol. 2016 Dec 1;134:1355-1363.
- Flitcroft DI. The complex interactions of retinal, optical and environmental factors in myopia aetiology. Prog Retin Eye Res. 2012 Nov;31:622-660.
- Walline JJ. Myopia Control: A Review. Eye Contact Lens. 2016 Jan;42:3-8.
- Li X, Friedman IB, Medow NB, Zhang C. Update on Orthokeratology in Managing Progressive Myopia in Children: Efficacy, Mechanisms, and Concerns. J Pediatr Ophthalmol Strabismus. 2017 May 1;54:142-148.
- Leo SW; Scientific Bureau of World Society of Paediatric Ophthalmology and Strabismus (WSPOS). Current approaches to myopia control. Curr Opin Ophthalmol. 2017 May;28:267-275.
- French AN, Morgan IG, Mitchell P, Rose KA. Patterns of myopigenic activities with age, gender and ethnicity in Sydney schoolchildren. Ophthalmic Physiol Opt. 2013 May;33:318-328.
- Chua SY, Sabanayagam C, Cheung YB, et al. Age of onset of myopia predicts risk of high myopia in later childhood in myopic Singapore children. Ophthalmic Physiol Opt. 2016 Jul;36:388-394.
- He M, Du Y, Liu Q, et al. Effects of orthokeratology on the progression of low to moderate myopia in Chinese children. BMC Ophthalmol. 2016 Jul 27;16:126.
- Lopes-Ferreira D, Ribeiroa C, Maiaa R, et al. Peripheral myopization using a dominant design multifocal contact lens. J Optom. 2011 Jan;4:14-21.
- Li SM, Kang MT, Wu SS, et al. Studies using concentric ring bifocal and peripheral add multifocal contact lenses to slow myopia progression in school-aged children: a meta-analysis. Ophthalmic Physiol Opt. 2017 Jan;37:51-59.
- Chan B, Cho P, Mountford J. The validity of the Jessen formula in overnight orthokeratology: a retrospective study. Ophthalmic Physiol Opt. 2008 May;28:265-268.
- Fu AC, Chen XL, Lv Y, et al. Higher spherical equivalent refractive errors is associated with slower axial elongation wearing orthokeratology. Cont Lens Anterior Eye. 2016 Feb;39:62-66.
- Mountford J. Rustin D. Trusit D. Orthokeratology: Principles and practice. Butterworth-Heinneman, 2004, p. 316.
- Liu YM, Xie P. The Safety of Orthokeratology—A Systematic Review. Eye Contact Lens. 2016 Jan;42:35-42.
- Charm J, Cho P. High myopia-partial reduction orthokeratology (HM-PRO): study design. Cont Lens Anterior Eye. 2013 Aug;36:164-170.
- Pauné J, Cardona G, Quevedo L. Toric double tear reservoir contact lens in orthokeratology for astigmatism. Eye Contact Lens. 2012 Jul;38:245-251.
- Kinoshita N. Konno Y. Hamada N. Kakehashi A. Suppressive effect of combined treatment of orthokeratology and 0.01% atropine instillation on axial length elongation in childhood myopia. Poster B0535- ARVO Baltimore. May 2017.
- Berntsen DA. Walline JJ. Mutti D. Swarbrick HA. Naidoo K. The Future of Myopia Management. Presentation at the Global Specialty Lens Symposium, Las Vegas, 2016.
- Gwiazda J, Thorn F, Held R. Accommodation, accommodative convergence, and response AC/A ratios before and at the onset of myopia in children. Optom Vis Sci. 2005 Apr;82:273-278.
- Gifford K, Gifford P, Hendicott PL, Schmid KL. Near binocular visual function in young adult orthokeratology versus soft contact lens wearers. Cont Lens Anterior Eye. 2017 Jun;40:184-189.





