The next patient on the schedule has an ocular prosthetic device. If you’re like the typical eyecare practitioner, the number of prosthetic patients in your practice is limited to a handful of infrequent encounters. Thinking back on your last prosthetics patient and your training in school, do you remember the best way to care for the patient in your chair?
Ocular prosthetic devices come in a variety of forms and are usually selected depending on the presence or absence of an eye. Those patients who have a globe present will be best fit with some form of prosthetic contact lens device, including tinted, print-transfer, or hand-painted soft contact lenses as well as larger scleral shells. Full-thickness reform eyes are typically the most natural-looking device and are customarily worn by anophthalmic patients. Regardless of the etiology and socket condition, it is important to be the patients’ advisor on how to clean the device and their ocular and/or socket tissues as well as on recommending polishing and replacement of the device accordingly.
SOFT PROSTHETIC CONTACT LENSES
Soft prosthetic contact lenses are a great addition to any eyecare practice, as a simple fitting set will allow practitioners to closely match a patient’s fellow eye. If attempting the hand-painted route, high-resolution digital photography and consultation with a manufacturer can enable color matching for both iris and scleral detail to an even higher degree.
These devices can be used to effectively mask scleral, corneal, lens, or iris deformities in sighted or non-sighted eyes. Additionally, they can be used therapeutically as an occlusion lens in young patients who have failed with patching therapy in the treatment of amblyopia, to eliminate the confusing image for patients who have diplopia, or to act as a comfort tint for patients who have conditions such as retinitis pigmentosa and ocular albinism. Figures 1 and 2 show examples of soft prosthetic contact lens options.

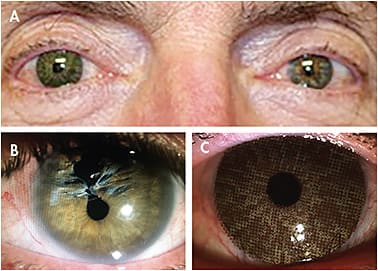
The process of fitting soft prosthetic contact lenses is relatively simple. Practitioners must first ensure that patients can maintain ocular health—and, in some cases, vision—while wearing a non-pigmented contact lens of the same material and fitting parameters. This process is similar to a general soft contact lens evaluation for fit, comfort, health, and vision.
For basic tinted and print-transfer lenses, initiate a color match to a patient’s good eye using either samples loaded onto a piston or a color matching card. The order of stacking of the samples is recorded and relayed to the lens manufacturer, and a new lens is printed and sent out for dispense.
For hand-painted lenses, a basic color match using a sample card or fitting set and high-resolution digital photography of the good eye is sent to the manufacturer. The manufacturer then sends back several trial lenses in varying hues of the patient’s basic eye color. The best trial lens for color matching is chosen, photographed for any modifications to color or details of the iris and sclera, and sent back for final fabrication. While it takes practice to confidently match patients’ eye color during the color matching process, it is a relatively straightforward and simple process once performed on a more frequent basis.
Ocular prosthetic soft contact lenses tend to be rated for one year of use, although there are some modalities that require more, or less, frequency of replacement. The most common reasons for replacement include adherence to manufacturer-recommended replacement frequency based on material, fading of the pigment over time with wear, or discomfort with the device. Although it is best to use the manufacturer’s recommended cleaning regimen, if the manufacturer of the device is unknown, a hydrogen peroxide system is a safe bet for hygiene and pigment preservation.
Dryness with ocular prosthetic contact lenses is similar to that of patients wearing other contact lens types. As the contact lens itself disrupts the tear film, rewetting drops may be required for comfortable wear. Any over-the-counter drop approved for use during contact lens wear—particularly non-preserved—will work well for maintaining comfort during device wear.
FULL-THICKNESS REFORM AND SCLERAL OCULAR PROSTHETIC DEVICES
For those patients who have atrophy of the globe or have completely lost an eye, a full-thickness reform eye or scleral shell eye may be the best option (Figure 3). These patients will have an eye made by a highly skilled ocularist over the course of one day to three weeks, with the resulting device having a near-exact cosmetic match to the fellow eye. In some eyecare practices, the eye itself is fit and fabricated in-house, but the artistry is performed outside of the office by an ocularist using high-resolution digital photography and color samples.

Examining Prosthetic Patients For the good eye, a prosthetic exam is no different from a general medical evaluation. In cases of a remaining atrophic globe, the examination is simple and familiar: evaluate each layer of the eye anteriorly to posteriorly under magnification either with or without dilation.
Practitioners seem to falter in evaluating the eye requiring an ocular prosthetic device, likely due to unfamiliarity with normal versus abnormal socket tissue presentations. Although the concern may be valid, there are still important aspects for socket health examination that should be monitored at an annual eye exam.
Therefore, in all cases of ocular prosthetic patient evaluations, the device should be removed from the socket to view the ocular tissues beneath. Patients oftentimes will remove the device themselves. In some cases, however, patients may require help if the device is not removed at home on a routine basis. Many eyecare practitioners know how to effectively remove a contact lens, but fewer are confident at inserting and removing rigid ocular prosthetic devices. See “Removal of an Ocular Prosthetic Device” on page 36 for general removal instructions.
Removal of an Ocular Prosthetic Device
- With your nondominant hand, attach a plunger or place thumb directly onto the center of the eye. Push upward gently to lift the device slightly in the socket.
- Beginning at the mid-temporal aspect of the lower lid, pull down the lid and push inward gently until the edge of the eye covers the lid.
- Slide your dominant hand medially to expose the entire inferior portion of the prosthetic device.
- Apply gentle downward pressure onto the device with your nondominant hand to slide the device into your dominant hand held below the socket.

The most important aspect of socket tissue evaluation is the general history gained from interviewing the patient. Although many prosthetic patients experience dryness and a mild amount of discharge throughout the day with ocular prosthetic wear, other potentially serious complications—such as bloody discharge, excessive mucus, and extreme socket sensitivity and/or pain—should be more carefully explored. Figure 4 reviews common socket tissue presentations.

In most cases of enucleation and evisceration, the remaining tissue within the socket is very similar to conjunctival tissue. Therefore, it can be afflicted by any potential disorders of conjunctival tissue. Patients may experience a conjunctivitis resulting from infection, inflammation, or mechanical irritation, with a presentation similar to any of these conditions.
Instillation of sodium fluorescein to evaluate for papillae, redness, conjunctival staining, and mechanical irritation can go a long way toward determining the etiology of a patient’s complaint, particularly when the offended area is in close apposition to a damaged or deposited area on the prosthetic device.
Any ocular disease state should be treated with the same topical therapy as other conjunctival infections. Consider an ointment or highly viscous vehicle for medication delivery because it may be more effective in treating the condition. Furthermore, applying the medication to the posterior aspect of the device prior to insertion may improve medication retention to the socket tissues.
Once staining or giant papillary conjunctivitis (GPC) is noted within the conjunctival tissues, the prosthetic device should be evaluated under magnification for scratches, deposits, chips, and delamination. In cases of an atrophic globe, areas of staining may require altering the fit of the device; this is true for both scleral shells and soft contact lenses. For enucleated or eviscerated eyes, modification or polishing of the device may be the easiest course of action, followed by replacement of the device.
The most commonly noted socket tissue reaction involves mechanical irritation of the tissues from the prosthetic device resting upon and rubbing against these areas. This may present with punctate epithelial staining, epiphora, or GPC. In these cases, a careful inspection of the prosthetic device and deep polishing in-office of deposits or imperfections on the device will help improve the socket tissue.
In addition to socket tissue complications arising from deposition on the device, the socket tissues themselves can become atrophied or the implant may migrate within the tissues. The most common complication due to implant migration or socket tissue atrophy is a poorly fitting ocular prosthetic device; the device may appear maligned, may rotate within the socket tissues, or may sit back further than the patient’s natural eye.
Any prosthetic device that fits poorly within the socket tissues may result in further irritation to the socket due to mechanical rubbing during wear; this will potentially increase discharge and may increase the patient’s susceptibility to infection or inflammation of the socket tissues. Therefore, evaluate the general location of the implant in addition to the socket tissues. The small sphere, made of acrylic, metal, or hydroxyapatite material, should be centered within the muscular cone, with either conjunctival graft tissue or sclera sewn above the implant. Any deviations in location or noted poor apposition of the prosthetic device to the socket tissues should be referred to an ocularist for refitting of the device.
On rare occasions, however, patients can suffer from a more serious implant complication such as extrusion. The process for this complication involves thinning of socket tissues and small changes in implant location within the socket, leading to evisceration of the overlying tissues. This complication occurs most commonly with patients who receive hydroxyapatite implants that do not have a protective coating; the coral-like backbone of the implant can become sharp over time and ultimately cut through the overlying tissues. These patients will routinely complain of increasing discharge either with or without a bloody color, but they typically do not complain of pain. If the face of the implant is showing through the tissue, is displaced far from center, or if any area appears to be extruded, the patient should be sent to an oculoplastics surgeon for an immediate evaluation for socket tissue reconstruction. Extrusion and implant migration are the most commonly missed etiologies for increased mucous discharge, infections, and pain with ocular prosthetic wear and can be caught with a simple socket tissue evaluation.
Following socket tissue evaluation with sodium fluorescein, rinse the patient’s socket thoroughly with non-preserved saline or eye wash. The device should be evaluated carefully for any deposits, scratches, chips, delamination, and other defects. The device can then be prepared for reinsertion into the socket by rinsing with non-preserved saline and coating with a non-abrasive GP solution or high-viscosity ocular lubricant. The sidebar titled “Insertion of an Ocular Prosthetic Device” on page 37 provides instructions on how to reinsert the rigid ocular prosthetic device.
Insertion of an Ocular Prosthetic Device
- Ensure any polish or compound has been removed from the prosthetic surface and wet with a non-abrasive GP cleaner prior to insertion of the device.
- Pin the upper lid to the brow bone with your nondominant hand.
- Orient the device so that the temporal portion of the eye is pointed superiorly (this generally allows comfortable insertion of the device by limiting the stretching of the lid tissues in the horizontal meridian).
- Gently slide the edge of the prosthetic device beneath the upper lid and secure in place with the thumb of the nondominant hand.
- With the dominant hand, reach below the device and evert the lower lid while simultaneously rotating the device with the nondominant thumb.

Modifications and Replacement Rigid ocular prosthetic devices should be replaced every three to seven years, although patients tend to extend this time for a well-fitting and cosmetically pleasing eye. Table 1 provides general guidelines for when to polish, modify, or replace an eye.
| Polish |
|
| Modify |
|
| Replace |
|
Observe the patient in primary and secondary gazes to reveal socket tissue atrophy or lid ptosis, which can be addressed with additional material on the existing eye or fabrication of a new device. Upon examination of the device with the slit lamp, the additional magnification can reveal chips, cracks, deep scratches, or delamination, which may require modifications to the patient’s device or fabrication of a new eye.
Modifications are performed with power tools and polishing and should be attempted only on plastic devices by a well-trained individual. The most common modification required is to enlarge the eye in some direction; the socket tissues tend to atrophy over time, leading to a ptotic lid, a sunken-in appearance, or rotation of the device within the socket tissues. This type of modification requires experience addressing each problem individually and is often best left to trained ocularists or to eyecare practitioners specializing in ocular prosthetic devices.
Due to the constant blinking over a plastic device, patients who have rigid ocular prosthetic devices tend to report dryness and depositing more compared to average GP wearers. A high-viscosity ocular lubricant used up to four times daily typically helps alleviate this discomfort. If over-the-counter lubricants are not acceptable for continued comfort during wear of the device, there are silicone-based lubricants formulated specifically for use with ocular prosthetic devices available for in-office sales through various companies.
Care and Maintenance Cleaning, care, and maintenance are typically an area of confusion for both patients and practitioners, so it is important to know the recommended cleaning, polishing, and replacement schedule for these devices (Table 2).
| OCULAR DEVICE | POLISHING FREQUENCY (PATIENT REMOVES OCULAR DEVICE AS DIRECTED) | POLISHING FREQUENCY (PATIENT DOES NOT REMOVE OCULAR DEVICE) | REPLACEMENT FREQUENCY |
|---|---|---|---|
| Scleral Cover Shell | 6 to 12 months | 3 months | 3 to 5 years |
| Reform | 6 to 12 months | 3 months | 5 to 7 years |
Just like rigid contact lenses, rigid ocular prosthetic devices are subject to surface scratches and deposits (Figure 5). The easiest and most effective method for at-home cleaning of these devices is with a non-abrasive GP cleaner, rinsing with saline, and then leaving dry when not worn. A thick ocular lubricant, prosthetic-specific silicone lubricant, or a small amount of a non-abrasive GP cleaner should be used to coat the device prior to reinsertion. Patients will typically leave a scleral cover shell out overnight, or they may remove a full-thickness reform prosthetic device daily or weekly.

Although the cleaning method described above works well for general at-home maintenance, some scratches and deposits need a heavier polish to improve the surface characteristics; typically, it is recommended that rigid devices should be polished every six to 12 months, with patients who remove the eye less frequently returning more often for heavy polishing.
A GP polishing unit is great for polishing all surfaces of the prosthetic device; however, more material will be removed if less polish and liquid is used as well as when a greater force is applied to the eye on the sponge. Removing too much material may result in accidental stripping of the clear coating, exposure and breakdown of the pigmented layers, and potential increased risk of chemical leeching from the pigmented layers. This may necessitate replacement of the device earlier than expected. Although encountered less frequently in the United States due to a U.S. Food and Drug Administration standard of fabrication, glass prosthetic eyes should never be polished with a GP modification unit, as they are highly susceptible to breaking during this process.
A less abrasive method of polishing a rigid ocular prosthetic device in the office is by applying a slurry of baking soda and hydrogen peroxide to both the anterior and posterior surfaces of the device. Rinse completely and soak in a non-abrasive GP cleaner for at least 15 minutes for optimal surface wetting.
IN CONCLUSION
Ocular prosthetic patients tend to be extremely loyal to practitioners who are willing and able to care for their socket health and ocular prosthetic device. These patients are not only a great way to mix up the day-to-day routine of eye care, but they also provide an excellent opportunity for growth as they tend to refer their entire circle of friends and family into the practice. Additionally, ocular prosthetic patients tend to arrive armed with a barrage of one-eye jokes, proving further that laughter truly is the best medicine. CLS




