An amniotic membrane is the innermost layer of the fetal membrane, which lines the inner cavity of the placenta.1 The amnion faces the fetus, and the chorion faces the uterus. Both layers are relatively avascular, metabolically active, and constantly restructure to accommodate the growing fetus.2 Over the past two decades, there have been thousands of publications on amniotic membranes and their use in health care.
OVERVIEW OF AMNIOTIC MEMBRANE CHARACTERISTICS AND USES
Amniotic membranes have anti-inflammatory, anti-fibrotic, anti-angiogenic, and anti-microbial properties. Also, because of their lack of immunogenicity and their ability to provide an effective substrate for growth, amniotic membranes are being used more and more for ocular surface reconstruction in a variety of ocular pathologies.1 It is incredibly important in such cases to control inflammation as soon as possible to prevent the cascade of events that can occur including scar tissue formation and disorganized scar tissue. The cornea requires the utmost clarity for optimal vision, so having an option to decrease the amount of scarring or haze is essential. As amniotic membranes lack immunologic markers, they are very well tolerated in a majority of patients, and the rejection rate is extremely low.
Natural amniotic membranes have been successfully used for wound and reconstructive purposes since the early 20th century. Human amniotic membranes have proven effective at expediting the healing process. The mechanism of action for these therapeutic effects is poorly understood but is thought to involve the resident growth factors present in near-term amniotic tissue.2 Amniotic membrane is rich in collagen and various growth factors that support the healing process.
Amniotic membranes improve wound closure and can also reduce or eliminate scar formation.3 Another unique property is the lack of immunologic markers, creating an “immune privileged” status on the allografts that results in a lower risk of tissue rejection.3 Amniotic membranes also contain antibacterial properties and have the ability to reduce pain on application.3 Furthermore, amniotic membranes have a high tensile strength.
Amniotic membranes contain cytokines, growth factors, structural proteins, and specialized proteins. The combination of these factors play an important role during fetal development, and it is thought that they may also play a critical role in repair and regeneration when applied to wounds.2
Amniotic tissue has many well-documented applications. This tissue is used for chemical and thermal burns, especially on third-degree skin burns. Many burn unit victims require amniotic membranes to protect their bodies and to expedite the healing process. Amniotic tissue is also used during reconstruction of the oral cavity and bladder as well as during tympanoplasty, arthroplasty, and for cases of omphalocele.4 Utilizing amniotic membranes can also prevent tissue adhesion during complex surgeries of the head, abdomen, pelvis, vagina, and larynx.4
Amniotic membranes are derived from placentas.5 The pregnant mother consents to the donation of her placenta after a scheduled caesarian section. There is rigorous testing during and after pregnancy to ensure that there are no communicable diseases such as human immunodeficiency virus (HIV), syphilis, hepatitis, etc.5 The mother is usually tested during pregnancy and after delivery, and the placenta tissue is tested at the processing facility multiple times. The mother and the donated tissue may also be tested months after the delivery to ensure that there are no diseases that arise later. However, although there have been no reported cases to date, acquiring a disease from human tissue will always remain a risk factor, and this should be reviewed with patients.
Who thought to use an amniotic membrane on the eye? The first ocular indication was suggested by de Rotth in 1940, who used it to successfully treat a chemical burn of the ocular surface.6 But it was with Juan Batlle’s report in 1992 that amniotic membrane usage became an important modality of treatment. There are currently more than 700 peer-reviewed publications for amniotic membrane ocular uses and applications.6
As research progresses, we are discovering that more and more ocular diseases are caused by inflammation, which is the first sign of wound healing. However, uncontrolled inflammation can lead to the following:
- Pain and discomfort
- Irritation
- Delayed healing, more tissue damage
- Vision-threatening complication, e.g., scar/haze
Effective control of inflammation is therefore an important strategy to promote quality healing and to prevent scar tissue/haze formation (or limiting the amount or appearance of scar tissue). As eyecare practitioners, we know the importance of keeping the cornea as clear as possible. Any ocular incident that could potentially lead to a corneal scar warrants prompt attention. Along with eye drops and other care regimens for treating ocular surface diseases, amniotic membranes should be considered to limit the inflammation and to reduce the amount of scar tissue.
It is thought that amniotic membranes neutralize the neutrophil activity and therefore downgrade the inflammation pathway.7 The reduction of inflammation decreases the likelihood of scarring.8 Heavy chain hyaluronic acids, which are also present in amniotic tissue, directly inhibit inflammatory cells.9 This can result in the suppression of T cell activation and can inhibit giant cell formation.
The addition of an amniotic membrane can result in:
- Anti-inflammatory benefits
- Promotion of limbal stem cell expansion
- Promotion of cellular migration
- Faster recovery
- Suppression of eye pain
AMNIOTIC TISSUE CLASSIFICATIONS
For ocular use, there are two types of amniotic tissue classifications: dry and wet.
Dry amniotic membranes are dehydrated in a way that preserves the key elements associated with healing. Different companies use their own proprietary methods to dehydrate the tissue; some use heat, others use air, others use chemicals, and others may use pressure. The tissue is packaged and stored at room temperature and has a two- to five-year shelf life depending on the manufacturer. Many companies offer dehydrated products.
Wet amniotic membranes are made by clipping a piece of amniotic membrane tissue between two rings made out of a clear, flexible material. They are cryo-preserved and are stored in a glycerol media to prevent freezing, which must be copiously rinsed off prior to application. Storage between 33º to 50º F will yield a shelf life of three months.5 When stored between 32º to –47º F (freezer), shelf life will increase to one year. When stored at even lower temperatures with commercial-grade freezers, the shelf life can be increased to two years. Because the tissue is stored in the glycerol media, the membrane is ready to be used immediately when removed from the freezer, with no thawing required.
Pros of dehydrated products:
- Less expensive for practitioners
- Long shelf life
- Easy storage (no refrigeration or freezer required)
- Can be more comfortable for patients
Pros of cryopreserved products:
- May retain more of the innate characteristics of the natural amnion, potentially resulting in better outcomes
- Cryopreserved membranes are cleared by the U.S. Food and Drug Administration (FDA) for wound healing rather than just for coverage (dry membranes)10
- Keeping extracellular matrix intact helps to regulate and promote tissue regeneration
CLINICAL CONSIDERATIONS
Ocular Uses Table 1 lists amniotic membrane uses in the ocular world. Adding an amniotic membrane to the management of any of these ocular conditions can result in quicker healing times, reduced scar tissue formation, decreased risk of corneal haze, and active healing. Amniotic membranes can also be excellent drug delivery devices.11
| • Pterygium surgeries |
| • High-risk corneal transplants |
| • Chemical burns |
| • Stem cell deficiencies |
| • Symblepharon |
| • Corneal ulcers |
| • Corneal abrasions |
| • Thermal burns |
| • Dry eye and exposure keratopathy |
| • Recurrent corneal erosions |
| • Filamentary keratitis |
| • Herpes keratitis |
When to Add an Amniotic Membrane Some practitioners opt to treat the ocular surface first with eye drops and ointments and then add an amniotic membrane a few days later. Other practitioners apply the amniotic membrane immediately. This decision depends on the individual practitioner and his or her comfort level; there is no right or wrong answer.
Supplies to Obtain Before Using Amniotic Membranes For dehydrated tissue, supplies needed include jewelers forceps, bandage contact lenses, pledgets, numbing drops (optional), and a lid speculum (optional). For cryopreserved tissue, supplies needed include numbing drops, gloves, saline solution, eye patching materials (optional), and blunt-tip or grooved forceps.
HOW TO APPLY AND REMOVE AN AMNIOTIC MEMBRANE
Applying a Dehydrated Amniotic Membrane There are two ways to apply a dehydrated amniotic membrane.
Method 1 Remove a bandage contact lens from its blister pack and place it on a clean surface. Carefully remove the amniotic membrane from its packaging with jewelers forceps, and gently place it inside the bandage contact lens (note: if using a product that is not dual sided, ensure that the proper side is face-down in the contact lens). Use a pledget to center the amniotic membrane and to remove air bubbles. Place the contact lens/amniotic membrane combination on your finger, and apply it directly onto the patient’s cornea. Have patients gently close their eyes for about three to five minutes for the membrane to properly adhere to the cornea. Afterward, evaluate with a slit lamp to ensure centration.
Method 2 Numb the patient’s eye with topical anesthetic. Use a lid speculum to keep the patient’s eye open. Remove the amniotic tissue from the packaging, and place the tissue directly onto the cornea. Use a pledget to press the tissue onto the cornea and to remove air bubbles. Then, place the bandage contact lens on the cornea. Gently remove the speculum, and have patients keep their eyes closed for three to five minutes. Afterward, evaluate with a slit lamp to ensure centration. (Note: in lieu of a speculum, practitioners may use the fingers from one hand to control the eyelids.)
Applying Cryopreserved Amniotic Tissue To apply cryopreserved amniotic membranes, first put on gloves, then remove the amniotic membrane from the packaging. Thoroughly rinse each side of the membrane with a copious amount of saline to rinse off the glycerol solution. This is an extremely important step; if you do not rinse off the glycerol media, it can burn and cause patient discomfort.
Once the membrane is thoroughly rinsed, numb the patient’s eye with topical anesthetic. Have the patient look down, and control his or her upper eyelid with your nondominant hand. Holding the membrane with your dominant hand, gently apply the top of it under the upper fornix; then, have the patient look straight ahead, and apply the lower portion of the membrane onto the lower eyelid fornix. Next, you can patch the eyelid with gauze or eye patching materials to improve comfort and to prevent dislodgement.
Removing a Dehydrated Amniotic Membrane To remove dehydrated amniotic membranes, simply remove the bandage contact lens carrier exactly as you would remove a bandage soft contact lens.
Removing a Cryopreserved Amniotic Membrane To remove cryopreserved amniotic membranes, first numb the patient’s eye with topical anesthetic. Have the patient look down. Lift his or her upper eyelid and, using blunt-tip forceps or specialized grooved forceps, grasp the edge of the polycarbonate ring and pull down and out to remove the device. Do not use jewelers forceps, as they will not firmly grasp the polycarbonate ring properly, leading to difficult removal.
PATIENT EDUCATION AND FOLLOW-UP INFORMATION
Eye Drop Schedule It is advisable to continue the same eye drop regimen that you would have prescribed even if a membrane was not used. So, if your patient was on antibiotic drops four times a day, consider keeping this same schedule.
Follow-Up Schedule The follow-up schedule will depend on the diagnosis and severity of the condition. For example, a mild dry eye patient may not require follow up for five to seven days, whereas a severe chemical burn may require follow up every 24 hours.
At the follow-up visits, some practitioners simply assess the ocular surface with the slit lamp without removing the device. I prefer to remove the device and to evaluate the cornea with fluorescein; but this is not absolutely necessary, and it depends on the patient case.
Patient Education It is advisable to explain the amniotic membrane technology to patients and to have them sign a consent form. The consent form should outline the risks and benefits so that patients are fully informed. Educate patients about any activity restrictions or visual expectations. In many cases, applying an amniotic membrane will blur patients’ vision (or they will have no vision if patching the eye), so it is important to notify patients of this. If patients have a party to attend or a vacation coming up, they may want to postpone the amniotic membrane application. Also, be sure to educate patients about any discomfort. It is quite normal for patients to have some mild ocular discomfort while the membrane is on the eye.
Reimbursement The proper code for amniotic membranes is 65778: Placement of an amniotic membrane on the ocular surface for wound healing; self-retaining. There is a zero-day global period. This means that if a patient comes in on day one and you apply an amniotic membrane, that patient can return the next day, and you can apply a brand new one and get reimbursed. You would not want to abuse this policy, but make a note for some of the most severe cases; there are some cases in which the ocular surface has absorbed the entire amniotic tissue and it is necessary to apply a new one the very next day. The most common conditions in which this may happen would include chemical and thermal burns, large corneal abrasions, and severe corneal ulcers/keratitis.
Paperwork As applying an amniotic membrane is considered a surgical procedure, a surgical note will need to be placed in the chart outlining the exact procedure used and comments about patient tolerance. All amniotic membranes come with a postcard or form to fax back to the company. The information required usually includes patient name, date of birth (DOB), contact information, practitioner name and location, diagnosis, and the serial number of the amniotic tissue. This is required to send back, because if there is a recall on that certain batch of tissue, the company will need to notify the affected practitioners and patients. This is how the company tracks this information for patient safety.
Cost Amniotic membranes vary in cost. Factors include dehydrated versus cryopreserved (dehydrated costs less), size of the membrane (e.g., a 12mm amniotic membrane usually costs more compared to a 5mm membrane), and quantity (if you order 20 amniotic membranes at a time, you may be able to get a better price than if you are only purchasing one). There are many different amniotic membrane companies now, so it may be a good idea to reach out to multiple companies to find out about their pricing.
REPRESENTATIVE CASES
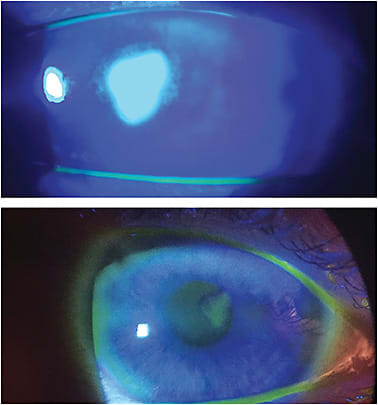
Case Report #1 A 54-year-old white female presented to the clinic complaining of a white spot on her left eye. The onset was two days previously, and she was experiencing pain, watering, photophobia, and decreased vision. She currently wore contact lenses and admitted to sleeping in them occasionally. She discontinued lens wear two days prior, when she first noticed the white spot on her eye. Her vision was 20/400 in the left eye and, upon slit lamp evaluation, a large central epithelial defect with microcystic edema was observed. The diagnosis was corneal abrasion/large central corneal ulcer.
The treatment was application of a dehydrated amniotic membrane along with fortified tobramycin every two hours and moxifloxacin every hour. She returned to the clinic every day for 24-hour follow-up visits. Each day, there was an improvement in the epithelial defect; the edema and the symptoms of pain and photophobia also improved. About two weeks later, the epithelial defect had resolved, and a very small scar remains (Figure 1). At this time, steroids were incorporated and then tapered.
Case Report #2 A 68-year-old white male presented to the clinic with a red, burning eye upon waking. He rated his pain as an 8 out of 10, and he also complained of photophobia, foreign body sensation, and epiphora. His ocular history was positive for laser-assisted in situ keratomileusis (LASIK) surgery in both eyes about 20 years ago. Upon slit lamp evaluation, a large central epithelial defect was discovered, presumably from dry eye (Figure 2A).
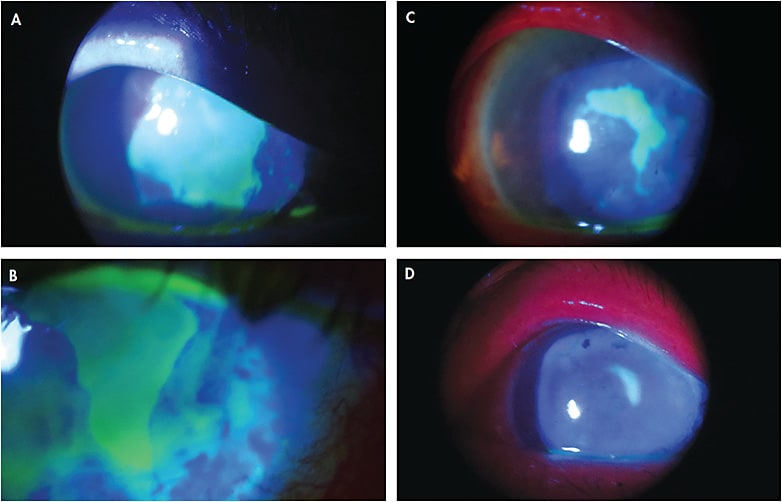
At this time, a bandage contact lens was applied along with topical antibiotics. The patient returned at regular intervals, but the epithelial defect was not responsive to any of the traditional treatments such as bandage contact lenses, antibiotic drops, artificial tears, ointments, and gels.
After about five days, we applied a cryopreserved amniotic membrane. We kept the eye drop regimen the same. After only three days, there was a significant improvement in the epithelial defect shape and size (Figure 2B). Over the course of one month, the eye continued to improve (Figures 2C and 2D). The membrane would dissolve every few days, and we used about six membranes in all for this patient. His defect eventually resolved completely, but some scar tissue and corneal irregularity remain. He now wears a scleral lens for the irregularity, but with the scleral lens, he is able to achieve 20/30 vision.
THE BOTTOM LINE
Amniotic membranes are best used on active, acute ocular situations. Conditions such as a corneal scar from 10 years ago will not respond to an amniotic membrane. The benefits of the tissue occur when the eye is still in the healing phase.
Amniotic membranes are not the end-all be-all cure for everything. A key pearl is to understand the underlying etiology of the condition. For instance, if a patient presents to the clinic with a chemical burn from acid that entered his or her eye, that is an acute condition, and it is likely that an amniotic membrane will assist in the healing process. On the contrary, if a patient has a non-resolving epithelial defect due to entropion, adding an amniotic tissue may heal the epithelial defect in the short term, but because the underlying condition is from the eyelid, the defect is likely to return. Therefore, an amniotic membrane will not provide a long-term solution until the eyelid is managed. Another situation would be dry eye. Many patients will do well with an amniotic membrane, reducing the ocular surface inflammation and healing epithelial defects; but dry eye is a chronic condition that requires constant management. Such patients will likely need to remain on their current eye drop and medication schedule long term.
Amniotic membranes offer practitioners an innovative technology to help treat certain ocular surface issues at a high level. Expediting healing, promoting cell migration, and reducing or eliminating scar tissue formation are all reasons to incorporate this incredible device into practice. It has become a new gold standard treatment in my practice, and I can’t imagine practicing without it. The results I have seen have been remarkable. The next time you have a patient who may benefit from an amniotic tissue, I hope that you reach for it. The outcome could pleasantly surprise you. CLS
REFERENCES
- Malhorta C, Jain AK. Human amniotic membrane transplantation: Different modalities of its use in ophthalmology. World J Transplant. 2014 Jun 24;4:111-121.
- Koob TJ, Lim JJ, Zabek N, Massee M. Cytokines in single layer amnion allografts compared to multilayer amnion/chorion allografts for wound healing. J Biomed Mater Res B Appl Biomater. 2015 Jul;103:1133-1140.
- Fetterolf DE, Snyder RJ. Scientific and clinical support for the use of dehydrated amniotic membrane in wound management. Wounds. 2012 Oct;24:299-307.
- Young RL, Cota J, Zund G, Mason BA, Wheeler JM. The use of an amniotic membrane graft to prevent postoperative adhesions. Fertil Steril. 1991 Mar;55:624-628.
- Adds PJ, Hunt CJ, Dart JK. Amniotic membrane grafts, “fresh” or frozen? A clinical and in vitro comparison. Br J Ophthalmol. 2001 Aug;85:905-907.
- Rahmen I, Said DG, Maharajan VS, Dua HS. Amniotic membrane in ophthalmology: indications and limitations. Eye (Lond). 2009 Oct;23:1954-1961.
- Magaña-Guerrero FS, Domínguez-López A, Martínez-Aboytes P, Buentello-Volante B, Garfias Y. Human Amniotic Membrane Mesenchymal Stem Cells inhibit Neutrophil Extracellular Traps through TSG-6. Sci Rep. 2017 Sep 29;7:12426.
- Mohammadi AA, Eskandari S, Johari HG, Rajabnejad A. Using Amniotic Membrane as a Novel Method to Reduce Post-burn Hypertrophic Scar Formation: A Prospective Follow-up Study. J Cutan Aesthet Surg. 2017 Jan-Mar;10:13-17.
- He H, Li W, Tseng DY, et al. Biochemical characterization and function of complexes formed by hyaluronan and the heavy chains of inter-alpha-inhibitor (HC*HA) purified from extracts of human amniotic membrane. J Biol Chem. 2009 Jul 24;284:20136-20146.
- McGaughy AG, Gupta PK. In-Office Use of Amniotic Membrane. EyeNet. 2015 Feb:31-32.
- Yelchuri ML, Madhavi B, Gohil N, Sajeev HS, Venkatesh Prajna N, Srinivasan S. In Vitro Evaluation of the Drug Reservoir Function of Human Amniotic Membrane Using Moxifloxacin as a Model Drug. Cornea. 2017 May;36:594-599.




