In the last few years, scleral lenses have become more and more prescribed because of their benefits. Based on this popularity, many designs are now available. Among them, sclerals of a large diameter are primarily prescribed to treat ocular surface disease, to manage severe eye dryness, or whenever practitioners’ clinical judgment dictates their usage. Lenses 17mm and larger represent almost 20% of the scleral lenses prescribed.1
At the same time, care regimen usage evolved as well. Hydrogen peroxide solution is now recommended for 35% of patients, mostly because it is considered a troubleshooting option for those presenting with discomfort issues, for its perceived higher disinfection and cleaning efficacies, for its convenience, or for those who need a preservative-free option.2 This is even more true among scleral lens prescribers; 61% of patients are provided with hydrogen peroxide to clean and store their scleral lenses.1
For those fitted with larger lenses, this may represent a challenge because sometimes the lenses do not fit in the case basket and, consequently, may break or be altered during the storage process. There are not many options available in the market to address this problem except to order larger contact lens cases online, subject to their availability. Other options may be more convenient for both patients and practitioners. One option is to use regular contact lens cases for scleral lens storage. The purpose of this study was to evaluate the impact on the scleral lens parameters of storing them with hydrogen peroxide in a non-neutralizing case for up to 30 days.
METHODS
This was a prospective study conducted after receiving Institutional Review Board (IRB) approval from the internal University ethics committee. Twenty new diagnostic scleral lenses (Boston XO material [Bausch + Lomb], 14.9mm diameter, base curve [BC] of 7.1mm to 8.4mm, power plano to –5.50D; all from Blanchard Labs) were cleaned and stored with hydrogen peroxide solutions in a regular non-neutralizing contact lens case for various lengths of time. Lenses were of the same design, and their power varied with the base curve to make sure that the profile was kept the same. For each lens, contact lens parameters were evaluated at baseline and after one, three, seven, and 30 days of non-interrupted storage; a washout period of dry storage was observed for 48 hours between each step. The researchers evaluated base curve with a radiuscope, diameter with a magnifier, and back-surface power with the use of a lentimeter.
Lens wetting angle (WA) was evaluated with a sessile drop technique just after storage (Figure 1). Briefly, each lens was mounted on a stand in front of a camera with high magnification. A single drop of non-preserved saline solution was placed on the surface with a calibrated syringe. The image was taken in front of a retro-illuminated white background (Figure 2). That image was then imported to a computer and processed with the National Institutes of Health’s imageJ software. Angle estimation was made with calipers built into the software (Figure 3). From every case, 2ml of hydrogen peroxide solution was extracted for each lens tested, and its concentration was measured through iodometric titration with sodium thiosulfate. Analysis was conducted at the chemistry department of the Université de Montréal.


A repeated measures ANOVA (analysis of variance) with one intragroup factor (time) was conducted to compare variations of the BC, lens power (P), WA, and peroxide concentration (PC) of the soaking solution over time.
RESULTS
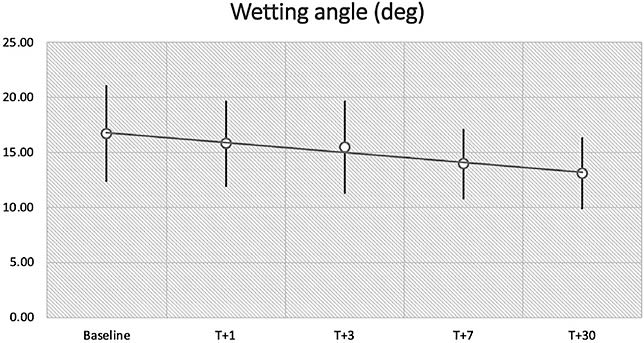
The evolution of the results is presented in Table 1. At baseline, the average lens P was –2.73D + 1.53D, with a BC of 7.68mm + 0.40mm. All 20 lenses were considered in the same group, assuming that they are made from the same design. These parameters (BC and P) did not change over time (F = 1.14; p = 0.38). However, the WA was significantly reduced after seven days (95% CI: 0.85; 4.71; P = 0.002) and more after 30 days (95% CI: 1.78; 5.40; P = 0.000), varying from 16.7º + 4.4º at baseline to 13.9º + 3.2º and 13.1º + 3.3º, respectively (Figure 4).
| BASELINE | T + 1 | T + 3 | T + 7 | T + 30 | |
|---|---|---|---|---|---|
| Power (D) | –2.73 + 1.53 | –2.73 + 1.53 | –2.73 + 1.53 | –2.73 + 1.53 | –2.70 + 1.51 |
| Base curve (mm) | 7.68 + 0.40 | 7.68 + 0.41 | 7.68 + 0.41 | 7.68 + 0.40 | 7.69 + 0.40 |
| Wetting angle (in degrees) | 16.7 + 4.4 | 15.8 + 3.9 | 15.4 + 4.2 | 13.9 + 3.2 | 13.1 + 3.3 |
| Peroxide concentration (%) | 3.01 + 0.02 | 3.06 + 0.02 | 3.03 + 0.05 | 3.04 + 0.07 | 3.05 + 0.02 |
DISCUSSION
The results of this study prove that it is feasible to keep scleral lenses in a non-neutralized peroxide solution for up to 30 days without affecting their parameters. Consequently, it may be possible to recommend storage in a non-neutralizing case (such as ones used for soft contact lenses), without modifying the lens fit, if parameters are kept unaltered.
The first question that may be raised relates to the fact that the peroxide is not neutralized during this process. True. Nonetheless, this is associated with many benefits. The first is the fact that the microbiota is exposed to 100% hydrogen peroxide during the entire soaking time. It represents a better disinfection process compared to the use of a neutralizing case, in which bacteria are exposed during 20 minutes—at the most—to 100% concentration of hydrogen peroxide. The second benefit comes from the surprising result that the lens surface improves its wettability over time as the lens stays in the peroxide solution. This is applicable only to the material tested, and under the conditions tested, but may represent a benefit for those patients who are struggling with chronic deposition on the lens surface.
The eye may be exposed to non-neutralized peroxide when lenses are applied on the eye. True. But the potential for significant exposure is likely to be minimal if not non-existent. We have to remember that scleral lenses are made of GP material. In the case of the one tested in this study, the manufacturer indicates that water absorption in the lens matrix is 1%. Therefore, except for the peroxide residue on the surface, chances of peroxide exposure through tear exchange when the lens is applied on the eye are very limited. As for residue adhering to the surface, a rinse with nonpreserved saline would eliminate the possibility of any exposure to peroxide during lens application.
The missing part of our study is the fact that we did not test this concept in a real-life scenario with scleral lens wearers and lenses that were contaminated with lipids and proteins. Thus, we cannot conclude from this study that the recommendation of using a non-neutralizing case is completely safe for patients. Anecdotally, I recommend this approach to patients fitted with sclerals of a large diameter that do not fit in the basket of neutralizing cases. To date, no adverse events have been documented as a consequence of peroxide exposure. This result is also duplicated in other practices, at least from discussions with other U.S. practitioners.
We also see this during orthokeratology follow-up visits. Patients are coming to their appointments with their lenses soaked in a neutralizing case, but for less than four hours (for morning consultations). After rinsing the lenses properly with saline solution, no adverse events happen when they are applied on the eye for assessment.
Another missing element is to determine whether long-term soaking in a non-neutralized peroxide solution may alter the resistance of the lens material to breakage. We do not believe that would be the case, however, because all parameters were kept unaltered during a 30-day storage period and there is no absorption of the solution in the lens matrix. This hypothesis will have to be tested in further studies. In the meantime, it may change the way that practitioners are storing their GP diagnostic lenses if it is proven that this is a better disinfection process.
CONCLUSION
Scleral lenses can be stored in a regular non-neutralizing contact lens case for a period up to 30 days with no modifications of their parameters. Longer storage may improve material wetting angle under certain circumstances. Knowing that the solution concentration remains high over 30 days of storage, these results may modify diagnostic lens storage habits. They may also influence recommendations to patients dealing with scleral lenses when their diameter exceeds the neutralizing case basket. Patients must be warned that this recommendation is an off-label procedure and that an efficient rinse must be performed before lens application.
Further clinical studies are needed to evaluate the potential side effects of using non-neutralized hydrogen peroxide to store scleral lenses on a larger population. CLS
Acknowledgement: Thanks to Anna Zarouk for her hard word collecting and analyzing data.
REFERENCES
- Schornack M. The Scope Study: An Overview. Contact Lens Spectrum. 2017 Dec;32:25-28.
- Nichols JJ, Fisher D. Contact Lenses 2018. Contact Lens Spectrum. 2019 Jan;34:18-23.




