When fitting scleral lenses, we may encounter a variety of roadblocks that can range from safely applying a lens on a patient who has a challenging condition to finding the source of a complaint and addressing complicated optics, just to name a few. Whether you’re new to scleral lenses or you have extensive experience, these tricks can be time-saving.
LENS APPLICATION CHALLENGES
Fitting processes vary among contact lens practitioners, particularly as they relate to lens application. Some practitioners have their assistants or technicians apply the first lenses, while others prefer to apply diagnostic lenses themselves. If a patient has ocular surface disease, the latter approach can be the highlight of your day, as you often will witness the reaction of patients who are suddenly relieved of their symptoms of severe pain and photophobia.
That said, lens application during the fitting process can be the biggest time drain of the appointment, during which much needs to be accomplished. There is pressure to obtain sufficient information to determine candidacy, assess the fit, apply the next lens (if necessary), and send the patient for optical coherence tomography or autorefraction, all while staying on time with the schedule for the day.
Let’s face it. No one wants a foreign object coming anywhere near his or her eye. No matter how prepared patients are for their appointments, they cannot overcome their own reflexes during lens application. Two common types of patients are the “squeezers,” who have a strong blepharospasm response, and the “rollers,” who have a strong Bell’s phenomenon. The worst case is the combination of the two. These reflexes can make appointments frustrating for practitioners and patients alike while the clock is ticking.
• The “Squeezers” Patients who have a strong blepharospasm response may include those who have no history of contact lens wear, those who are experiencing pain, and children. Sometimes, it just happens. The key for practitioners is to be in complete control of the lens. Not only are patients squeezing their eyelids, they may flinch and knock the lens to the floor. When fitting these patients, you could try to apply the lens as quickly as possible, which is not always successful, or you could desensitize the reflexes.
Desensitizing reflexes takes time and can be tricky, but it is feasible, and it’s an investment in a patient’s confidence while he or she is learning to be independent. You can break down the application process into steps as follows:
- Help the patient become familiar with someone else’s hands holding his or her eyelids open. During this step, there is no lens, and typically, patients start to relax and to squeeze less.
- Move the lens into the patient’s line of sight. Do not fill the lens with saline, as this shows patients that you won’t apply it during this step. Patients become accustomed to seeing the lens while the eyelids are held open, and they know it’s safe.
- Fill the lens with saline, and reassure the patient that it will not be applied to the eye. Hold the lens on the lower eyelid, and when the patient is relaxed, tip the lens so that the eye gets wet. The patient feels the plastic of the lens and the liquid in preparation for the actual lens application.
When working with a child, offer to stay at the same step, go back a step, or proceed to the next step throughout the process. Even if you have a great opportunity to apply the lens when “practicing,” you should not do so without notifying the patient. Applying the lens when patients aren’t ready may defeat the purpose of desensitizing the reflexes, and you will lose patients’ trust. Once patients are comfortable with the process, lens application should be easy.
Another trick when patients have a strong squeezing reflex is to tell them to wiggle their toes immediately before applying the lens. During that brief moment of surprise, a patients’ guard drops, and the practitioner or technician has the perfect opportunity to apply the lens.
• The “Rollers” Patients who have a strong Bell’s phenomenon may be challenging as well. Their eyes roll up as soon as a lens is near. Giving a patient a stuffed animal or a stress ball to hold can provide a stimulus that will guide both eyes downward to his or her hands. If the practitioner’s hand that is holding the lens does not block the contralateral eye, the patient can continue viewing the target to conquer Bell’s phenomenon. Simply telling patients to look down and keep their eyes open doesn’t always work.
Patients may experience Bell’s phenomenon when applying the lenses themselves, and unfortunately, the cornea can be injured during a failed attempt (Figure 1). Patients can cut a plunger and place a light beneath it or use a commercially available lighted plunger and stand to help maintain fixation while applying a lens.
SYMPTOMS AFTER LENS APPLICATION
All symptoms of extreme discomfort during the fitting process should be carefully investigated. Scleral lenses are known to be comfortable, but patients may sometimes experience focal discomfort. Often, this is related to a lifted edge interacting with the eyelids.1
To determine whether edge lift is causing a patient’s discomfort, pull the eyelids away from the edges of the lens. If the symptom goes away, then the lens edge is likely lifting off somewhere. When this is the case, reassure patients that the fit can be enhanced by making lens modifications. The fitting process can be scary for patients, and reassurance is instrumental in scleral lens success.
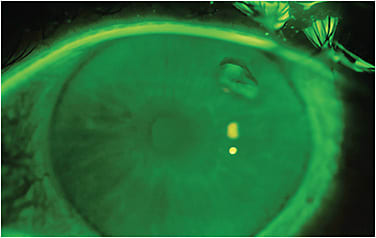
Fluorescein or lissamine green can help you visualize edge lift where the dye seeps under the lifted edge (Figure 2). Sometimes the edge lift, even if significant, is parallel to the surface of the sclera or conjunctiva, and the dye may not pool because of a large gap. In this situation, directing illumination toward the side of the presumed lifted edge will create a shadow indicative of edge lift (Figure 3). If an air meniscus exists, patients will definitely be uncomfortable (Figure 4).



Edge lift should be lowered, steepened, or tightened depending on the terminology used by the lens manufacturer. Circumferential edge lift should be adjusted around the entire lens. Meridional edge lift may require toric landing zones or haptics. If the edge lift is located in one quadrant, ask whether the manufacturer can make quadrant-specific changes to the edges.
While edge lift is one of the most common reasons for discomfort after initial lens application, other factors that are unique to scleral lenses can also affect comfort. These include:


- Application Bubbles Bubbles are easy to identify without a slit lamp; however, a large bubble that occupies the entire vaulted reservoir of the lens can be deceiving, because the outline of the bubble is hidden by the lens curvature (Figure 5). Patients may be symptomatic until the lens is reapplied without a bubble. Application bubbles are great learning opportunities for patients to become independent with their lenses and to learn how to avoid bubble trouble in the future.
- Tight-Fitting Lenses Typically, tight-fitting lenses are comfortable when first applied and become increasingly uncomfortable over time. A too-tight lens will cause obvious compression and impingement of the vessels and conjunctiva under the lens as well as redness surrounding the edges (Figure 6).
- Corneal Touch Although patients may be uncomfortable with corneal touch, they may not report significant discomfort with excessive touch as they would with edge lift.
THE PAIN-WITHOUT-STAIN PHENOMENON
What about patients who jump out of the chair in pain immediately after a lens is applied? The lens looks great. It vaults nicely with no bubbles. You detect no edge lift, compression, or impingement. Why is the patient reacting so strongly? Much less common than the issues that I previously discussed—but far more insidious—is neuropathic pain.
Many patients find their way to scleral lens providers because of significant dry eye and pain. These patients may have exposure keratopathy, graft-versus-host disease, Sjögren’s, or other forms of severe dry eye. Typically, they present with dense corneal staining, low Schirmer test scores, conjunctival staining, and decreased or nonexistent tear lakes and tear breakup times. These are all signs of severe ocular surface disease.
Another category of patients reports significant dry eye symptoms even though their tear lake looks normal and they have no evidence of staining. Based on the symptoms alone, practitioners will try scleral lenses. A subset of these patients experience extreme pain and discomfort immediately after the diagnostic lens is applied. These patients are likely experiencing neuropathic pain.
Ocular pain, interpreted as dryness, was first described by Belmonte as aberrant nerves after refractive surgery causing “phantom” corneal pain similar to phantom limb pain.2 Rosenthal and colleagues coined the phrase “pain without stain” to describe individuals who had significant ocular symptoms with few or no signs.3 Symptoms include burning, stinging, aching, and light sensitivity, all of which are common to dry eye, and yet there are no signs.4 These patients experience hyperalgesia—or a heightened pain response—to benign stimuli such as moving air, temperature changes, light, and scleral lenses.
In this population, with little to no ocular pathology and considerable symptomatology, ruling out lens-fitting issues first is crucial. Examining these patients may be difficult, as they just want the lens removed, but an examination is in their best interest to determine how to alleviate their pain.
Neuropathic pain can arise from peripheral nerves, central nerves, or both.4 Some patients who have peripheral neuropathic pain may do well with scleral lenses, so it’s important to assess the lens thoroughly before giving up. A quick way to determine whether the pain is peripheral or central is a proparacaine challenge test.4 If the topical anesthetic eliminates a patient’s symptoms, the pain is peripheral. These patients may do well with scleral lenses, but if they are hypersensitive to the lenses or edges, bandage soft contact lenses may alleviate their symptoms. If the topical anesthetic mildly decreases a patient’s symptoms, the pain may emanate from both peripheral and central nerves, and scleral lenses may not alleviate it. If the pain is central, the anesthetic may not make a difference or it may worsen the pain. In this situation, scleral lenses may stimulate pain. These patients may benefit from systemic medications that reduce abnormal activity in injured nerves. This topic is beyond the scope of this article.
In summary, if you suspect neuropathic pain, try the topical anesthetic challenge to help determine whether scleral lenses may be an option. If a patient experiences some relief with a scleral diagnostic lens and wishes to proceed, be forewarned that this may be a challenging fit with many remakes, because patients who have hyperalgesia are sensitive to even the smallest lens adjustments.
OPTICAL TRICKS: THE ROLE OF THE RETINOSCOPE
As the name implies, irregular corneas come in all shapes and sizes. GP optical correction, whether with corneal or scleral lenses, can mask an irregularity but not always and not completely. In large part, this is because the optics mask the anterior-surface aberrations, while the posterior-surface aberrations remain. Therefore, residual higher-order aberrations may persist.5-10 Not every practice has an aberrometer, but many practices have retinoscopes. A retinoscope can be a valuable tool and a poor man’s aberrometer in a pinch.
When performing retinoscopy on patients who have myopia, hyperopia, or astigmatism (low-order aberrations), the streak of light directed at the eye will bounce off the retina and provide a linear reflex unless it is already neutralized. In that case, the entire pupil is filled evenly with reflexed light. In keratoconic eyes, the retinoscopy shows a scissors reflex, likely because of higher-order aberrations. You might hope that a scleral lens will correct all of the higher-order aberrations so that only easily corrected, low-order aberrations remain. Unfortunately, that is not the case. Scissors reflexes may occur with a scleral lens on the eye.11 Figure 7 is an example of a retinoscopic image through a scleral lens on a keratoconic eye. The reflex does not resemble a uniform streak. In this case, retinoscopy was used to refract centrally in the cone and in the area surrounding the cone, and there was a 2.50D difference, depending on the starting point of the retinoscopy refraction. This patient exhibited a multifocal cornea, which skewed the autorefractor reading.

Figure 8 shows another example of multifocal optics in a patient who has Marfan syndrome and a subluxated lens. This patient achieved 20/20 visual acuity with a scleral lens with two different over-refractions. With a base curve of 7.10mm and –2.00D power, the first over-refraction was +7.75D, but the patient reported poor near vision. The second over-refraction was –10.00 –4.50 x 045, providing 20/20 distance and near visual acuity. The retinoscope helped determine the second over-refraction, as the autorefractor kept resulting in errors.

Try Different Base Curves To boost your scleral lens fitting success, take the time to familiarize yourself with your fitting set(s), and discuss any questions with the laboratory consultants. Sometimes, the lens that provides optimal clearance does not provide the best possible vision. Having an extensive fitting set or multiple sets with different base curves can be advantageous.
Not every lens design can uncouple the base curve from the sagittal depth. For example, some designs require steep base curves to achieve large sagittal depths, whereas other designs can maintain the same sagittal depth regardless of whether the base curve is steep or flat. Several designs have oblate (with flat base curves) and prolate (with steep base curves) options meant for fitting different corneal topographies; yet, these lenses can also be utilized to improve optics.
Unlike corneal GP lenses in which the fitting curve is the base curve, scleral lenses have much more flexibility. There is no reason why an oblate lens cannot be applied to a highly ectatic cornea. But why would you do this? If a patient has a clear cornea without scarring and the pinhole acuity is better compared to the scleral lens-corrected acuity, aberrations are likely at work. This is a case in which a retinoscope may be helpful. Different base curves can provide better and worse acuity. Anecdotally, some practitioners have found that vision can be improved simply by changing the base curve. Flatter base curves on ectatic corneas often (but not always) can improve a patient’s corrected visual acuity.
Chair time is precious, and you need not spend too much time over-refracting each lens with different base curves. Apply the lens and check the retinoscopy reflex. The more linear the streak and the less scissoring, the more likely that you will achieve better endpoint visual acuity with that diagnostic lens. Begin the over-refraction at that time. If scissoring increases, try a different base curve.
The beauty of rigid lens optics is the rule of SAM/FAP (steep add minus/flat add plus). A scleral lens with a 7.0mm base curve and –6.00D power can be made with an 8.0mm base curve and plano power and still correct a patient’s vision. One may or may not provide a better endpoint, depending on the eye and the aberrations. Also, if a lens is highly plus-powered and thick, a steeper base curve can thin the lens by the same optical principles of SAM/FAP.
EXPAND YOUR FITTING SET WITH CUSTOM DESIGNS
One size does not fit all when it comes to scleral lenses, and custom designing additional diagnostic lenses for your repertoire will help speed up your fitting process. Consider adding some lenses with flat peripheral curves, steep peripheral curves, highly toric peripheral curves, or any other shape that you commonly encounter in practice. If you have found that flat base curves optically help patients who have ectasias, you can design diagnostic lenses with deeper sagittal depths and flat base curves to accommodate varying degrees of ectasia. There are always patients who are outliers. Consider purchasing a scleral profilometry instrument to aid in fitting complex scleral shapes, or seek training in scleral lens molding technology.
ALLEVIATE SYMPTOMS WITH INFORMED, EFFICIENT CARE
In an ideal situation, one diagnostic lens would cure all. That said, patients who need scleral lenses are not in an ideal situation, and they need help. Seamless application and removal of diagnostic lenses can accelerate the fitting process, particularly if optics are an issue and require multiple diagnostic lenses. Understanding patients’ symptoms and determining whether they stem from the lens fit or from a pathology will enable you to treat patients who are suffering with pain. Exploring all options and being efficient with quick tricks will make all the difference. CLS
REFERENCES
- Barnett M, Johns LK. Contemporary Scleral Lenses. Ophthalmology: Current and Future Developments. 2017, Sharjah: Bentham Science Publishers Ltd, pp.199-240.
- Belmonte C. Eye Dryness Sensations After Refractive Surgery: Impaired Tear Secretion or “Phantom” Cornea? J Refract Surg. 2007 Jun;23:598-602.
- Rosenthal P, Baran I, Jacobs DS. Corneal Pain without Stain: Is it Real? Ocul Surf. 2009 Jan;7:28-40.
- Goyal S, Hamrah P. Understanding Neuropathic Corneal Pain--Gaps and Current Therapeutic Approaches. Semin Ophthalmol. 2016 Mar;31:59-70.
- Chen M, Yoon G. Posterior Corneal Aberrations and Their Compensation Effects on Anterior Corneal Aberrations in Keratoconic Eyes. Invest Ophthalmol Vis Sci. 2008 Dec;49:5645-5652.
- Choi J, Wee WR, Lee JH, Kim MK. Changes of Ocular Higher Order Aberration in On- and Off-Eye of Rigid Gas Permeable Contact Lenses. Optom Vis Sci. 2007 Jan;84:42-51.
- Kosaki R, Maeda N, Bessho K, et al. Magnitude and Orientation of Zernike Terms in Patients with Keratoconus. Invest Ophthalmol Vis Sci. 2007 Jul;48:3062-3068.
- Marsack JD, Parker KE, Pesudovs K, Donnelly WJ 3rd, Applegate RA. Uncorrected Wavefront Error and Visual Performance During RGP Wear in Keratoconus. Optom Vis Sci. 2007 Jun;84:463-470.
- Negishi K, Kumanomido T, Utsumi Y, Tsubota K. Effect of Higher-Order Aberrations on Visual Function in Keratoconic Eyes with a Rigid Gas Permeable Contact Lens. Am J Ophthalmol. 2007 Dec;144:924-929.
- Tomidokoro A, Oshika T, Amano S, Higaki S, Maeda N, Miyata K. Changes in Anterior and Posterior Corneal Curvatures in Keratoconus. Ophthalmology. 2000 Jul;107:1328-1332.
- Johns LK. Retinoscopy to the Rescue. I-Site Newsletter June 2017. Available at http://netherlens.com/june_2017 . Accessed June 15, 2020.




