When fitting contact lenses, it is fundamental to use vital stains, also referred to as dyes, to assess ocular surface integrity and to evaluate GP contact lens fitting on the eye. To evaluate the corneal tissue and the fitting relationship for corneal GP lenses, sodium fluorescein (NaFl) is the most indicated dye because of the high contrast of its orange color with the colored iris. Given contact lens practitioners’ many years of experience with fitting corneal GP lenses, they have developed the habit of using NaFl for their fitting evaluation, and they tend to continue using this dye to also evaluate the scleral lens fitting relationship with the underlying ocular surface.
While NaFl may be used when fitting scleral lenses to determine whether the lens is bearing on the cornea and to estimate the depth of the fluid reservoir, it may not be the appropriate dye to assess the lens fit over the scleral zone, because its orange color has a reduced contrast with the white sclera. Thus, the most indicated dye to assess the scleral area is lissamine green because of the higher contrast of its dark green color with the white color of the sclera. With these considerations, I believe that both of these dyes are needed in scleral lens practice. Table 1 summarizes the characteristics of both dyes.
| VITAL DYE CHARACTERISTICS | FLUORESCEIN | LISSAMINE GREEN |
|---|---|---|
| Color | Orange | Green |
| Illumination | Cobalt blue —————————————— White |
White |
| Filter | Wratten 11, 12, 15, 47, or 47A —————————————— Tiffen yellow |
None ———————————— Wratten 92 Hoya 25A |
| Ocular surface staining area | Cornea | Conjunctiva |
| Time of observation | Immediately to 8 minutes | Immediately to 1–4 minutes |
SODIUM FLUORESCEIN
NaFl is essential when fitting scleral lenses to detect lens bearing in the corneal and limbal areas, to evaluate the depth of the tear fluid reservoir, and to assess the corneal tissue integrity after lens removal.
Instilling NaFl in the bowl of the scleral lens along with the preservative-free saline is important to determine whether the lens is properly vaulting the cornea and the limbus. This can be achieved using a magnification of 6x to 16x and diffuse illumination with a cobalt blue light. Dark areas indicate lens bearing on the cornea and/or the limbus. The use of a yellow filter (Wratten 11, 12, 15, 47, or 47A or Tiffen yellow) positioned within the observation system will enhance contrast, facilitating the observation. The NaFl in the bowl may also be used to more accurately estimate the depth of the tear fluid reservoir in the corneal and limbal area. This can be achieved by using the optic section technique (with the slit beam rotated approximately 45º), white light, and a magnification of 10x to 25x while the eye is gazing straight ahead. The depth of the tear fluid reservoir can then be estimated by comparing the thickness of the fluid reservoir with the known scleral lens thickness.
At lens removal, NaFl staining is used to evaluate corneal epithelium integrity. NaFl stains spaces in which cells are missing, damaged, or altered, even in normal eyes.1-3 The amount of time that it takes for corneal staining to appear may vary among patients and can range from immediately to eight minutes after NaFl instillation, depending on various factors such as patients’ tear volume and turnover as well as ocular surface conditions.4 The eye is assessed in five different gaze directions—straight, superior, inferior, nasal, and temporal—using the diffuse illumination technique with a cobalt blue light and a yellow filter. A magnification of 10x with a broad beam may be used for an overall assessment; use up to 40x magnification to detect more understated staining as well as the staining depth.
LISSAMINE GREEN
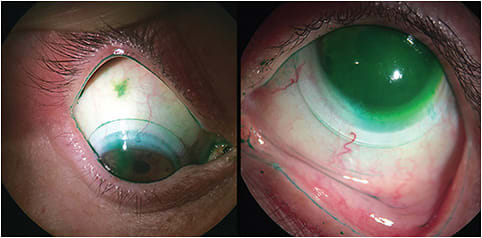
Velasquez has suggested the use of lissamine green with scleral lenses, stating that it is very practical.5 Lissamine green should be used to better evaluate scleral lens alignment on the conjunctival tissue, highlighting the lens periphery and/or edge lift, scleral toricity, any influx of the stained fluid into the tear reservoir (Figure 1), conjunctival impingement (Figure 2), and conjunctival tissue integrity (Figure 3), because its green color has a higher contrast with the white sclera. Lissamine green staining is observed with white light, with which it appears as a blue-green stain, or with the use of a red filter such as Hoya 25A or Wratten 92, which enhances the red barrier that lissamine green absorbs, appearing as a black-colored stain.



To evaluate the scleral lens alignment with the conjunctiva, assess the eye in the five different gaze directions with a magnification of 6x to 25x, using diffuse illumination straight-on or rotating the slit beam 90º. The edge lift will appear as a dark band or shadow under the lens edge. The parallelepiped or direct focal illumination techniques may also be used. Excessive pooling of stained liquid under the lens edge and/or the periphery will appear when the lens is lifting off from the ocular surface.
To assess conjunctival staining, the eye should be evaluated in the five different gaze directions using the diffuse illumination technique with a magnification of 6x to 25x. There is consensus that lissamine green stains damaged and dead conjunctival cells.1 It may also be used to evaluate Marx’s line when assessing for lid margin staining such as lid wiper epitheliopathy (Figure 4). Lissamine green is rapidly absorbed by the conjunctiva; therefore, the staining should be observed within four minutes of instillation.1,6,7 In cases of dry eye, the time of absorption and the visibility of staining may be prolonged.4
MIXTURE OF DYES
With both dyes being beneficial when fitting scleral lenses, is it possible to instill them concurrently in the eye? It has been shown that the mixture of 2% NaFl and 1% lissamine green resulted in optimal staining without irritation.8 Korb et al, using the two dyes at the same time, did not find a cancellation effect. The use of different lights will help to evaluate the two dyes separately; as discussed earlier, cobalt blue light will enhance the appearance of NaFl, and white light will enhance the appearance of lissamine green. However, lissamine green will not have an impact on estimating the depth of the tear reservoir when using white light.
The dyes are available in liquid and in strips. Liquid dyes provide better delivery, but they are not easily found and are expensive. Dye strips are the most commonly used, and grading scales recommend them. CLS
REFERENCES
- Bron AJ, Argüeso P, Irkec M, Bright FV. Clinical Staining of the Ocular Surface: Mechanisms and Interpretations. Prog Retin Eye Res. 2015 Jan;44:36-61.
- Mocan MC, Irkec M. Fluorescein Enhanced Confocal Microscopy in Vivo for the Evaluation of Corneal Epithelium. Clin Exp Ophthalmol. 2007 Jan-Feb;35:38-43.
- Wilson G, Ren H, Laurent J. Corneal Epithelial Fluorescein Staining. J Am Optom Assoc. 1995 Jul;66:435-441.
- Begley C, Caffery B, Chalmers R, Situ P, Simpson T, Nelson JD. Review and Analysis of Grading Scales for Ocular Surface Staining. Ocul Surf. 2019 Apr;17:208-220.
- Velasquez H. Lentes esclerales: haciendo facil lo complejo, lente de prueba inicial y apticas toricas. Presentation presented at FEDOPTO. Bogota, Colombia, 2017, Aug. 4-6.
- Hamrah P, Alipour F, Jiang S, Sohn JH, Foulks GN. Optimizing Evaluation of Lissamine Green Parameters for Ocular Surface Staining. Eye (Lond). 2011 Nov;25:1429-1434.
- Foulks GN. Old and New Methods of Evaluating the Ocular Surface. Ocul Surf. 2014 Jan;12:1.
- Korb DR, Herman JP, Finnemore VM, Exford JM, Blackie CA. An Evaluation of the Efficacy of Fluorescein, Rose Bengal, Lissamine Green, and a New Dye Mixture for Ocular Surface Staining. Eye Contact Lens. 2008 Jan;34:61-64.




