Contact lenses offer a tremendous opportunity to provide an exceptional visual experience for many of our patients. The multitude of options available in soft, GP, and hybrid designs allows clinicians to optimize both fit and vision characteristics of the lenses to achieve success with those patients who are interested in wearing contact lenses.
While contact lenses provide many opportunities and benefits to our patients, they also can create some challenges. Contact lenses are significantly thicker compared to the tear film, resulting in new dynamics on the ocular surface and creating a pre-lens tear film. The material characteristics, wetting properties, and lens design can significantly influence the comfort and performance of the lens. Innovations in these areas have advanced the wearing experience significantly. However, it is critical to understand the influence of a contact lens on the ocular surface.
One of the major concerns with contact lens wear is the potential disruption of ocular surface homeostasis. Corneal epithelial dendritic cell density has been found to be increased in the midperipheral cornea of contact lens wearers when compared to non-contact-lens wearers in younger ages.1 Contact lens discomfort has been linked with increased levels of inflammation; this results in increased levels of nerve growth factor, leukotriene B4, peroxidation products, phospholipase A2, interleukin 17-A, and substance P as well as in degraded lipids.2,3
The ocular surface is a complex system that requires the appropriate tear film constituents to allow the lid wiper to interact appropriately with the cornea and conjunctiva. The lipid, aqueous, and mucin layers of the tear film must be delicately balanced to provide the appropriate level of lubrication; this keeps the coefficient of friction between the lid wiper and the ocular surface as low as possible.
The tear film is critical for protecting the ocular surface. The relationship between the tear film breakup time (TBUT) and the interblink interval (IBI) is referred to as the ocular surface index (OSI). The OSI is calculated by dividing the TBUT by the IBI. A normal OSI is greater than 1.0, which provides the ocular surface with the protection of an adequate tear film.4 The IBI tends to be shorter for dry eye patients, which may serve as a compensatory mechanism to keep the ocular surface protected.5 Several factors can alter the IBI. As an example, individuals viewing a computer screen for extended periods of time have been shown to have a longer IBI, increasing exposure of the ocular surface between blinks and adding to ocular surface challenges.5
When the glands that produce the tear film function appropriately, the three layers of the tear film work in a coordinated way to provide a homeostatic state for the ocular surface. The three layers are:
- Lipid Layer The thin, anterior surface of the tear film secreted by the meibomian glands and by the glands of Zeiss and Moll. It functions to retard evaporation of the tear film between blinks and promotes tear film stability.
- Aqueous Layer The thick, central section of the tear film, it is reflexly secreted by the lacrimal gland and basally by the glands of Wolfring and Krause. It functions to lubricate the ocular surface while maintaining a high optical quality and a neutral pH.
- Mucin Layer The thin, mucoid layer located adjacent to the cornea that is formed by the goblet cells of the conjunctiva and is rich in glycoproteins.
A properly functioning tear film consists of a complex molecular interaction that involves the constituents of all three layers. As opposed to three distinct layers, it is a delicate molecular transition of layers that is required to maintain a homeostatic ocular surface state.
OCULAR SURFACE INFLAMMATION
Ocular surface inflammation associated with contact lens wear is important to appreciate in our efforts to optimize contact lens comfort. Understanding appropriate strategies to optimize ocular surface health through managing inflammation is critical with contact lens wearers to help optimize long-term wearing success.
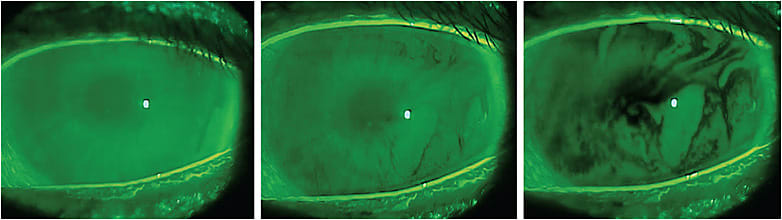
Several ocular surface signs give us clinical evidence of abnormalities in the tear film, including the presence of corneal and conjunctival staining with lissamine green (LG) and fluorescein (FL), lid wiper epitheliopathy (LWE), and a reduced tear film breakup time (TBUT) (Figure 1). These findings have been shown to be associated with ocular surface inflammation.7 Several other clinical diagnostic tests designed to assess the integrity of the ocular surface include meibography, meibomian gland evaluation, tear osmolarity test, Schirmer’s test, phenol red thread test, and matrix metalloproteinase-9 (MMP-9) test. These provide the opportunity to assess the ocular surface and to identify abnormalities in structure and function as well as biochemical alterations of the ocular surface over time.
With one test that measures MMP-9 on the ocular surface, a red line appears in the results window if the test detected MMP-9 greater than 39 ng/ml. If a red line is not present, the test is negative, and MMP-9 levels are lower than 39 ng/ml.8
The positive signal strength varies in intensity based on the concentration of MMP-9 collected from the ocular surface; darker positive signals (red lines) are associated with higher concentrations of MMP-9 collected. Brujic and Kading presented a grading scale that divides the signal strength into five categories based on how dark the positive signal strength is: negative, trace positive, weak positive, positive, and strong positive9 (Figure 2). This not only determines the relative level of inflammation at the time of diagnosis, it also provides the opportunity to monitor relative inflammation levels to determine the influence of treatment on ocular inflammation.

INFLAMMATORY CONDITIONS ASSOCIATED WITH CONTACT LENS DISCOMFORT
There are several conditions that can affect comfortable contact lens wear. Identifying these conditions and treating them appropriately will ultimately provide opportunities for improved lens wear. Not treating them may lead to inflammation that can eventually reduce successful contact lens wear. Here we will review some of the more common conditions associated with chronic inflammation and how to successfully treat them to optimize the contact lens-wearing experience.
Ocular Allergies Ocular allergies are associated with increased levels of inflammation. There are several types of allergic eye disease. Seasonal and perennial allergic conjunctivitis conditions are driven by an allergen-immunoglobulin E (IgE) response that triggers mast cells to degranulate. This results in a significant release of histamine that causes itching, conjunctival blood vessel dilation, tearing, and swelling of the ocular tissues. Prolonged exposure to the allergen will cause additional recruitment of other chronic inflammatory cells and increased levels of inflammatory proteins.
Identification of these patients is critical, as these individuals often come into the office for their yearly examination at a time when they have no symptoms or clinical signs of allergic conjunctivitis. Arming them with the appropriate tools to help them manage the condition when they do experience symptoms is critical. Often, these patients are trying to manage their symptoms on their own. They do this by either discontinuing lens wear or by using over-the-counter drops that they have selected for themselves without appropriate professional guidance.
Treatment strategies to minimize inflammation initially include optimizing the properties of contact lenses. This includes ensuring the appropriate fit, selecting materials that optimize ocular surface homeostasis, and selecting replacement schedules that optimize patients’ wearing experience. If a patient’s prescription is available in a daily disposable lens, this will be ideal so that the lens can be discarded at the end of the day. This reduces the opportunity for re-exposure to allergens that may not be removed with cleaning from a reusable lens. Refitting patients with daily disposable lenses can improve palpebral signs and reduce symptoms of allergic conjunctivitis.10
For those patients whose prescription is not available as a daily disposable lens, cleaning and disinfecting with a hydrogen peroxide-based system works well to ensure appropriate cleaning and disinfection of the lens. Recent research has demonstrated that 3% hydrogen peroxide cleaning and disinfection solutions are extremely effective at removing pollen from soft contact lenses.11
Coupling appropriate lens selection with a therapeutic approach is critical for some patients to optimize clinical outcomes. For seasonal allergic conjunctivitis, the best options are those therapeutics that provide antihistamine and mast-cell-stabilizing properties. Fortunately, these patients have several options available either over-the-counter (OTC) or by prescription including ketotifen 0.025%, olopatadine (0.1%, 0.2%, and 0.7%), epinastine (0.05%), azelastine (0.05%), bepotastine (1.5%), alcaftadine (0.25%), and cetirizine (0.24%). Recently, the first ketotifen-eluting daily disposable contact lens was approved in Japan12 and in Canada to alleviate allergic conjunctivitis symptoms for people wearing contact lenses. Some of these listed pharmaceuticals have been shown to improve contact lens comfort.13,14
Often, these pharmaceuticals work well in controlling the signs and symptoms of allergic eye disease, because they control the response at the root of the problem by inhibiting mast cell degranulation and providing antihistamine activity. Additional inflammation can arise and may require topical corticosteroids. Loteprednol 0.2% is approved for the clinical signs and symptoms of ocular allergies to be utilized up to four times a day.
Other types of allergic disease including vernal, atopic, and giant papillary conjunctivitis recruit high levels of inflammatory cells and can impact successful contact lens wear. Lid eversion is critical in your contact lens wearers (Figure 3) to ensure a healthy palpebral conjunctival surface. Each of these conditions can adversely affect the conjunctiva, creating a poor lens-wearing experience for patients. Appropriate control of the inflammation is critical in these individuals.

This will typically require antihistamine/mast-cell-stabilizing agents, but often this isn’t sufficient. Topical corticosteroids are often pulsed for brief periods of time to reduce inflammation, and then the inciting stimulus for the development of the condition should be removed. For many ocular surface allergies, daily disposable contact lenses would benefit the patients’ wearing experience. Hydrogen peroxide is my preferred cleaning system for those patients who are not candidates for daily disposable lenses.
Floppy Eyelid Syndrome Floppy eyelid syndrome (FES) is a condition in which low levels of elastin are present in the eyelids, causing low pressure of the lids against the globe. While sleeping, spontaneous eversion of the eyelids can occur, causing exposure of the ocular surface including the cornea, bulbar conjunctiva, and palpebral conjunctiva. This exposure creates elevated levels of inflammation on the ocular surface and can lead to corneal and conjunctival staining, conjunctival hyperemia, papillary conjunctivitis, mucous discharge, and significant ocular discomfort for patients.
There is a strong association between the presence of FES and sleep apnea. If these individuals are being treated for sleep apnea, they are often using continuous positive airway pressure (CPAP). During sleep, the seal between the instrument and the skin can loosen, causing air to be forced toward the eyes, drying their eyes. This dryness can affect the eyes during the day and reduce comfortable contact lens wear.
Initial treatment includes covering the eyes overnight with eye shields. If patients have difficulty wearing eye shields, they can attempt bland ointments to cover any exposed parts of the ocular surface while sleeping. Corrective eyelid surgery to help with eyelid laxity can significantly improve patient symptoms that cannot be controlled by other options.
Anterior Blepharitis Blepharitis presents as increased inflammation of the lid margins and ocular surface. This condition is critical to identify in contact lens wearers. Anterior blepharitis has a variety of etiologies, with bacteria, Demodex, seborrhea, and rosacea being the most common.
Traditional examination techniques have typically guided us to view the lid margin at a low magnification. I have found that viewing the lid margin at a higher magnification and having patients look down has exposed inflammation and deposits at the lid margin that are often undetected if this technique is not performed.
Overpopulation of the microorganisms that cause deposits at the base of the lashes can create inflammation at both the lid margin and at the ocular surface. Microbial overgrowth releases excessive exotoxins, increasing the likelihood of ocular discomfort.15
Acute treatment strategies involve reducing inflammation and reducing quantities of the microorganisms that are present in excessive populations. For bacterially driven blepharitis, combination antibiotic-steroid drops provide an appropriate treatment to reduce both overpopulation and inflammation. Antibiotic/steroid combinations have been shown to produce better clinical outcomes for blepharitis compared to antibiotics alone.16 Commercially available formulations include tobramycin 0.3%/loteprednol 0.5%, tobramycin 0.3%/dexamethasone 0.1%, and neomycin/polymyxin B/dexamethasone 0.1%.
Contemporary formulations include sophisticated delivery vehicles that provide increased tissue residence time, resulting in increased tissue penetration and enhanced antibiotic efficacy. Tobramycin 0.3%/dexamethasone 0.05% was produced with a xanthan gum vehicle, which is an advanced delivery mechanism that allows these properties at lower corticosteroid levels.17 When prescribing these combination medications for blepharitis, I typically advise patients to place the drops directly on the eyes and then rub excess drops on the lid margin.
Demodex overpopulation is critical to identify, as these mites are resistant to traditional antibiotics and require an alternative therapy. Tea tree oil applied to the lid margin has been shown to reduce Demodex populations there. The active ingredient in tea tree oil that provides activity against Demodex is terpinen-4-ol. A recent in vitro study exposed human meibomian gland epithelial cells to terpinen-4-ol and showed a concentration- and time-dependent decrease in cell survival.18 Although in vitro doesn’t always translate to what occurs in vivo, carefully observe and monitor meibomian gland health when treating Demodex with tea tree oil.
One company is investigating a new therapeutic drop for the treatment of Demodex blepharitis. TP-03 0.25%, dosed two times per day, showed substantial improvements in Demodex populations at the lid margin when compared to placebo controls.19 If approved, this medication will be the first to be indicated for Demodex blepharitis.
Microblepharoexfoliation (MBE) physically removes debris and biofilms from the lid margin. It is an in-office procedure in which a microsurgical-grade sponge is soaked in a soap solution and then rotated at high velocity along the lid margin, reducing the floral population.
Research has demonstrated improvements in ocular surface health after MBE. In a small study, 10 subjects who had positive MMP-9 tests underwent a single MBE procedure. After one month, all had demonstrated improvement in both Ocular Surface Disease Index (OSDI) scores and in TBUT, and all had a negative MMP-9 test result.20
Long-term management of the lid margin health is critical after initial treatment. Several commercially available wipes exist that feature anti-microbial activity along with surfactants to help prevent collarettes from forming on the lid margin. Hypochlorous acid, available at various concentrations, also works well to control bacterial populations at the lid margin. Research has demonstrated a significant reduction of the lid microflora 20 minutes after the application of 0.01% hypochlorous acid to the lid margin.21
Meibomian Gland Dysfunction Meibomian gland dysfunction (MGD) can affect the ocular surface through inadequate production of the lipid layer. The altered lipid layer may adversely affect contact lens comfort. MGD can vary in its severity and in its presentation. Obvious MGD, as its name implies, describes the clinical presentation in which the MGD is obvious based on slit lamp evaluation. This may include lid margin hyperemia, tylosis, madarosis, visible capping of glands, and serrated lid margins. Non-obvious MGD is the clinical presentation in which the lid margin looks relatively quiet, but inadequate meibum is produced from the glands, usually because of obstruction of meibum at the orifice. To identify these patients, pressure must be applied to the lid margin to identify the lack of meibum from the glands. This can be performed in a controlled manner utilizing a meibomian gland evaluator (MGE).
MGD has been shown to increase inflammation on the ocular surface. Elevated levels of tear inflammatory cytokines TNF-alpha, IL-6, and IL-8 have been found in patients who have MGD.22 All of these challenges resulting from MGD create a non-optimized ocular surface to support contact lens wear. Fortunately, there are several treatment options available to optimize the lens-wearing experience for these patients.
A 12-minute thermal pulsation treatment is approved for MGD. A single procedure has been shown to improve meibomian glands yielding secretions (MGYS), TBUT, and dry eye symptoms nine months after the treatment. Additionally, contact lens wearers demonstrated an additional four hours of comfortable contact lens-wearing time.23
With another device to treat MGD, clinicians provide treatment to the lid margin by simultaneously applying heat and pressure. This device allows the lid margin to be visualized and the meibum to be actively expressed. Improvements in meibomian gland function, TBUT, and OSDI scores were demonstrated after a single treatment.24
Another device provides heat to the outside of the lids through smart lid applicators for 12 minutes, followed by manual expression. Practitioners can customize the manual expression. It is critical to understand where the MGs have dropped out to know which regions of the lid margin to avoid. This procedure has resulted in improvements in meibomian gland secretions, corneal staining, TBUT, and subjective symptoms of dry eye.25
Intense pulsed light (IPL), when applied to the skin, causes blood cells in the abnormal telangiectasias to absorb the light, coagulate, and close the blood vessels. IPL treatment has been shown to improve TBUT, corneal staining, lid margin irregularity, lid thickness, meibomian gland function, and OSDI scores and to reduce the incidence of positive MMP-9 immunoassay.26
MGD is critical to identify in contact lens wearers, as it can be associated with ocular surface inflammation and with contact lens discomfort. It is key to treat these patients appropriately to improve the quality of meibum being secreted and to ultimately optimize the lipid layer of the tear film.
Dry Eye It is critical for the ocular surface to be healthy and functioning appropriately to optimize the likelihood of successful contact lens wear. Dry eye and, in particular, the ocular surface inflammation associated with dry eye will make comfortable contact lens wear more difficult. As such, it is critical to identify and to appropriately reduce inflammation. It can be helpful to measure MMP-9 levels on the ocular surface as a biomarker for inflammation. Fortunately, when elevated, we have several options to treat ocular surface inflammation in those wearing contact lenses.
Cyclosporine A is a calcineurin inhibitor that exerts immunomodulatory effects by blocking T-cell infiltration, activation, and the subsequent release of inflammatory cytokines. It was approved to increase tear production in patients whose tear production is presumed to be suppressed due to ocular inflammation associated with keratoconjunctivitis sicca. Cyclosporine has been shown through impression cytology to reduce inflammatory markers on the ocular surface, and it increases goblet cell numbers and improves patient symptoms.27,28 A small study examining contact lens wearers reported that patients utilizing cyclosporine 0.05% b.i.d. OD and OS for five weeks increased comfortable lens-wearing time and overall contact lens comfort.29 Another study demonstrated no difference in lens wearers randomized to cyclosporine 0.05% b.i.d. OD and OS or to placebo for three months.30 The contradiction in these studies demonstrates the complex nature of contact lens comfort and the importance of selecting appropriate candidates to begin cyclosporine treatment.
More recently, a 0.09% formulation of cyclosporine, Cequa (Sun Pharmaceuticals) has become available. In addition to the higher concentration, its nanomicellular formulation favors dispersion and solubility of cyclosporine A into the precorneal tear film. Cyclosporine 0.09%, dosed twice a day, demonstrated improvements in conjunctival staining, corneal staining, and tear production.31
Lifitegrast is available as a 5% concentration in unit-dose vials and is approved for the treatment of the clinical signs and symptoms of dry eye disease. Lifitegrast is a lymphocyte function-associated antigen-1 (LFA-1) antagonist. It prevents the interaction of LFA-1 on activated T-cells from interacting with intercellular adhesion molecule-1 (ICAM-1) that are over-expressed on the surface of inflamed ocular surface tissues.32 A retrospective analysis demonstrated that lifitegrast 5% reduced the percentage of patients testing MMP-9 positive.33 Additionally, lifitegrast 5% has demonstrated reduced corneal staining and reduced dry eye symptoms over a three-month time period.34
Oral ingestion of essential fatty acids (EFAs) has become a valuable component in helping to control inflammation on the ocular surface. A recent placebo control trial with re-esterified omega-3 fatty acids taken for three months demonstrated statistically significant improvement in tear osmolarity, omega-3 index levels, TBUT, MMP-9 levels, and OSDI symptom scores.35 Another randomized double-masked control trial demonstrated improvements in OSDI, TBUT, and lid margin inflammation from ingesting 1.5 grams of omega-3 fatty acids.36
Corticosteroids have been used to manage inflammation associated with dry eye for decades. Recently, a 0.25% loteprednol formulation, Eysuvis (Kala Pharmaceuticals), was approved for the short-term management of the clinical signs and symptoms of dry eye. It contains a mucus-penetrating particle (MPP) technology, branded as Ampplify, that are 300nm in size, allowing it to penetrate more efficiently into targeted ocular surface tissues. It is approved to be used four times a day for up to two weeks. It has demonstrated improvement in both symptoms and conjunctival hyperemia.37 Important to note is that it will be difficult to wear contact lenses during treatment, as it is not approved for use in conjunction with contact lens wear. Minimizing contact lens wear for the two weeks of treatment may be advisable if the treatment is appropriate for a given patient.
SUMMARY
Inflammation can impact a contact lens wearers’ ability to wear contact lenses successfully. As such, it is critical for clinicians to determine the underlying cause of inflammation and to treat appropriately to ensure that it doesn’t reduce comfortable lens wear. When we appropriately identify and treat the cause of ocular surface inflammation, only then can we win not only the battle but also the war. CLS
REFERENCES
- Golebiowski B, Chao C, Bui KA, Lam WYW, Richdale K, Stapleton F. Effect of age and contact lens wear on corneal epithelial dendritic cell distribution, density, and morphology. Cont Lens Anterior Eye. 2020 Feb;43:84-90.
- Willcox MD. Is There a Role for Inflammation in Contact Lens Discomfort? Eye Contact Lens. 2017 Jan;43:5-16.
- Gad A, Vingrys AJ, Wong CY, Jackson DC, Downie LE. Tear film inflammatory cytokine upregulation in contact lens discomfort. Ocul Surf. 2019 Jan;17:89-97.
- Ousler GW 3rd, Hagberg KW, Schindelar M, Welch D, Abelson MB. The Ocular Protection Index. Cornea. 2008 Jun;27:509-13.
- Johnston PR, Rodriguez J, Lane KJ, Ousler G, Abelson MB. The interblink interval in normal and dry eye subjects. Clin Ophthalmol. 2013;7:253-259.
- Schlote T, Kadner G, Freudenthaler N. Marked reduction and distinct patterns of eye blinking in patients with moderately dry eyes during video display terminal use. Graefes Arch Clin Exp Ophthalmol. 2004 Apr;242:306-312.
- Yang S, Lee HJ, Kim DY, Shin S, Barabino S, Chung SH. The Use of Conjunctival Staining to Measure Ocular Surface Inflammation in Patients With Dry Eye. Cornea. 2019 Jun;38:698-705.
- Sambursky R, Davitt WF 3rd, Latkany R, et al. Sensitivity and specificity of a point-of-care matrix metalloproteinase 9 immunoassay for diagnosing inflammation related to dry eye. JAMA Ophthalmol. 2013 Jan;131:24-28.
- Brujic M, Kading D. Making Matrix Metalloproteinase-9 Levels More Meaningful. Presented at the Global Specialty Lens Symposium January, 2016, Las Vegas.
- Solomon A. Allergic manifestations of contact lens wearing. Curr Opin Allergy Clin Immunol. 2016 Oct;16:492-497.
- Sunaga T, Mimura T, Matsuoka H, Horikawa H, Kitsu K, Mizota A. Is Hydrogen Peroxide Disinfection Effective for Cleaning Pollen Particles Attached to Contact Lenses? Clin Optom (Auckl). 2020 Aug 20;12:123-128.
- Johnson & Johnson Vision. Johnson & Johnson Vision Receives Approval of World’s First and Only Drug-Releasing Combination Contact Lens for Vision Correction and Allergic Eye Itch: ACUVUE® TheravisionTM. Press Release. 2021 Mar 24. Available at https://www.prnewswire.com/news-releases/johnson--johnson-vision-receives-approval-of-worlds-first-and-only-drug-releasing-combination-contact-lens-for-vision-correction-and-allergic-eye-itch-acuvue-theravision-with-ketotifen-301254442.html . Accessed June 8, 2020.
- Brodsky M, Berger WE, Butrus S, Epstein AB, Irkec M. Evaluation of comfort using olopatadine hydrochloride 0.1% ophthalmic solution in the treatment of allergic conjunctivitis in contact lens wearers compared to placebo using the conjunctival allergen-challenge model. Eye Contact Lens. 2003 Apr;29:113-116.
- Nichols KK, Morris S, Gaddie IB, Evans D. Epinastine 0.05% ophthalmic solution in contact lens-wearing subjects with a history of allergic conjunctivitis. Eye Contact Lens. 2009 Jan;35:26-31.
- Rynerson JM, Perry HD. DEBS - a unification theory for dry eye and blepharitis. Clin Ophthalmol. 2016 Dec 9;10:2455-2467.
- Torkildsen GL, Cockrum P, Meier E, Hammonds WM, Silverstein B, Silverstein S. Evaluation of clinical efficacy and safety of tobramycin/dexamethasone ophthalmic suspension 0.3%/0.05% compared to azithromycin ophthalmic solution 1% in the treatment of moderate to severe acute blepharitis/blepharoconjunctivitis. Curr Med Res Opin. 2011 Jan;27:171-178.
- Scoper SV, Kabat AG, Owen GR, et al. Ocular distribution, bactericidal activity and settling characteristics of TobraDex ST ophthalmic suspension compared with TobraDex ophthalmic suspension. Adv Ther. 2008 Feb;25:77-88.
- Chen D, Wang J, Sullivan DA, Kam WR, Liu Y. Effects of Terpinen-4-ol on Meibomian Gland Epithelial Cells In Vitro. Cornea. 2020 Dec;39:1541-1546.
- Karpecki P, Ceballos J, Massaro-Corredor M, et al. Safety and Efficacy of TP-03 for the Treatment of Demodex Blepharitis: Mars and Jupiter Trials. Presented at Annual Meeting of the American Academy of Optometry (virtual), Nov. 2020.
- Connor CG, Narayanan S, Miller W. Reduction in inflammatory marker matrix metalloproteinase-9 following lid debridement with Blephex. Presented at the 2017 ARVO Annual Meeting, May 2017, Baltimore.
- Stroman DW, Mintun K, Epstein AB, et al. Reduction in bacterial load using hypochlorous acid hygiene solution on ocular skin. Clin Ophthalmol. 2017 Apr 13;11:707-714.
- Zhao H, Li Q, Ye M, Yu J. Tear Luminex Analysis in Dry Eye Patients. Med Sci Monit. 2018 Oct 24;24:7595-7602.
- Blackie CA, Coleman CA, Nichols KK, et al. A single vectored thermal pulsation treatment for meibomian gland dysfunction increases mean comfortable contact lens wearing time by approximately 4 hours per day. Clin Ophthalmol. 2018 Jan 17;12:169-183.
- Tauber J, Owen J, Bloomenstein M, Hovanesian J, Bullimore MA. Comparison of the iLUX and the LipiFlow for the Treatment of Meibomian Gland Dysfunction and Symptoms: A Randomized Clinical Trial. Clin Ophthalmol. 2020 Feb 12;14:405-418.
- Karpecki P, Wirta D, Osmanovic S, Dhamdhere K. A Prospective, Post-Market, Multicenter Trial (CHEETAH) Suggested TearCare System as a Safe and Effective Blink-Assisted Eyelid Device for the Treatment of Dry Eye Disease. Clin Ophthalmol. 2020 Dec 30;14:4551-4559.
- Lee H, Han YE, Park SY, et al. Changes in the expression of matrix metalloproteinase-9 after intense pulsed light therapy combined with meibomian gland expression in moderate and severe meibomian gland dysfunction. Cont Lens Anterior Eye. 2021 Jun;44:101339.
- Brignole F, Pisella PJ, De Saint Jean M, Goldschild M, Goguel A, Baudouin C. Flow cytometric analysis of inflammatory markers in KCS: 6-month treatment with topical cyclosporin A. Invest Ophthalmol Vis Sci. 2001 Jan;42:90-95.
- Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002 Mar;120:330-337.
- Hom MM. Use of cyclosporine 0.05% ophthalmic emulsion for contact lens-intolerant patients. Eye Contact Lens. 2006 Mar;32:109-111.
- Willen CM, McGwin G, Liu B, Owsley C, Rosenstiel C. Efficacy of cyclosporine 0.05% ophthalmic emulsion in contact lens wearers with dry eyes. Eye Contact Lens. 2008 Jan;34:43-45.
- Goldberg DF, Malhotra RP, Schechter BA, Justice A, Weiss SL, Sheppard JD. A Phase 3, Randomized, Double-Masked Study of OTX-101 Ophthalmic Solution 0.09% in the Treatment of Dry Eye Disease. Ophthalmology. 2019 Sep;126:1230-1237.
- Perez VL, Pflugfelder SC, Zhang S, Shojaei A, Haque R. Lifitegrast, a Novel Integrin Antagonist for Treatment of Dry Eye Disease. Ocul Surf. 2016 Apr;14:207-215.
- Soifer M, Mousa HM, Stinnett SS, Galor A, Perez VL. Matrix metalloproteinase 9 positivity predicts long term decreased tear production. Ocul Surf. 2021 Jan;19:270-274.
- Sheppard JD, Torkildsen GL, Lonsdale JD, et al. Lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease: results of the OPUS-1 phase 3 study. Ophthalmology. 2014 Feb;121:475-483.
- Epitropoulos AT, Donnenfeld ED, Shah ZA, et al. Effect of Oral Re-esterified Omega-3 Nutritional Supplementation on Dry Eyes. Cornea. 2016 Sep;35:1185-1191.
- Oleñik A, Jiménez-Alfaro I, Alejandre-Alba N, Mahillo-Fernández I. A randomized, double-masked study to evaluate the effect of omega-3 fatty acids supplementation in meibomian gland dysfunction. Clin Interv Aging. 2013;8:1133-1138.
- Korenfeld M, Nichols KK, Goldberg D, et al. Safety of KPI-121 Ophthalmic Suspension 0.25% in Patients With Dry Eye Disease: A Pooled Analysis of 4 Multicenter, Randomized, Vehicle-Controlled Studies. Cornea. 2021 May;40:564-570.




