Contact lens prescribing in the United States is transforming. Sales of mass-produced contact lenses continue to migrate online, with more consumers using online contact lens prescription renewal and delaying in-office professional eye care. More consumers are purchasing lenses using their smartphone, even without a valid prescription.
The underlying influences include Federal Trade Commission (FTC) regulations, advances in consumer technology and e-commerce, the globalization of business, shifting consumer behavior, and COVID-19. It is difficult to pinpoint the relative contribution of regulatory trade decisions alone, but this is undoubtedly influential, even if intertwined with other factors. Following is a review of the U.S. contact lens regulatory landscape and a look at what industry observers see ahead.
AN OVERVIEW OF U.S. CONTACT LENS PRESCRIPTION RELEASE
The first mass-marketed frequent replacement contact lens launched in the late 1980s. Prescribing practitioners were initially the exclusive sellers of these lenses. As the mass-produced contact lens market grew with new lens offerings, alternative distribution emerged. The first online sellers of contact lenses entered the market in the mid-1990s. At the time, few practitioners charged contact lens service fees, which meant that their revenue came solely from the sale of product. When consumers filled their contact lens prescriptions outside of their eyecare practitioners’ offices, prescribers lost their profit margin. Hence, prescribers were reluctant to release contact lens prescriptions. The FTC viewed withholding contact lens prescriptions as anticompetitive and unfair to consumers and consequently mobilized to provide relief.
In 2004, the FTC promulgated the Contact Lens Rule (the Rule), pursuant to the Fairness to Contact Lens Consumers Act (FCLCA), which mandated automatic contact lens prescription release to allow consumers the ability to shop for their contact lenses. Additionally, it mandated that prescribers are to verify contact lens prescriptions to sellers, and sellers are to provide contact lenses only in accordance with a valid prescription. As part of the ongoing systematic review of all FTC rules and guides, in 2015 the Commission requested public comments pertaining to several topics including the economic impact and benefits of the Rule; any possible conflicts between the Rule and state, local, or other federal laws or regulations; and the effect of any technological, economic, or other industry changes on the Rule.1
After an extensive review by the FTC, with consideration of—from 2015 to 2019—thousands of public comments and materials that included surveys, studies, analyses, and information generated at an FTC workshop devoted to the Rule and to the evolving contact lens marketplace,2 the FTC determined that there was inadequate prescriber compliance with automatic contact lens prescription release. The Commission approved a final rule amending the Contact Lens Rule to further facilitate shopping for contact lenses, requiring prescribers to have patients confirm receipt of their prescription. The Final Rule went into effect on Oct. 16, 2020, but it was a false start. Two months later, an omnibus bill passed by Congress delayed the implementation and enforcement of the Final Rule until March 31, 2021, but without undoing the amendments to the FCLCA. In the bill, the House Committee on Appropriations expressed that:
“The Committee is disappointed that the FTC’s final amendments to the Contact Lens Rule do not sufficiently address the patient safety concerns the Committee has repeatedly outlined in report language for the past four years. The rule fails to sufficiently modernize the prescription verification process by eliminating the use of robocalls and imposes new burdensome paperwork requirements on providers and patients. To allow providers sufficient time to implement the necessary changes and prevent additional interruptions in service due to the coronavirus, the Committee directs the FTC to delay the effective date for the amendments, and to suspend any implementation or enforcement of those amendments, until March 31, 2021.”3
Although the amendments are referred to as “final,” the FTC has signaled that it has not completed the process of evaluating the contact lens space. When the FTC announced the amendments to the agency’s Contact Lens Rule on June 23, 2020, Federal Trade Commissioner Rebecca Kelly Slaughter issued a concurrent statement4 in which she observed, “…an anticompetitive dynamic in the contact lens market in the United States that cannot be fixed within the bounds of our rulemaking authority under the current Act.” She suggested that Congress explore removing specification of a manufacturer on contact lens prescriptions. She also wrote, “Another path that may prove fruitful is for Congress to task the [U.S. Food and Drug Administration (FDA)] with conducting a study about the therapeutic interchangeability of different kinds of lenses for common ocular ailments, such as nearsightedness. The FDA could also study whether a minimum prescription period of two years instead of one year would benefit consumers without threatening ocular health.”
In essence, the FTC is telegraphing intent to investigate contact lens prescription interchangeability and lengthened expiries. It is uncertain whether these directions are valid, as they may violate FDA regulations and infringe on intellectual property, among other concerns.
Meanwhile, the American Optometric Association (AOA) is supporting the Contact Lens Rule Modernization Act, introduced by Sen. John Boozman (R-AR) to the Senate in September 2020.5 This bill seeks to replace the signed acknowledgement requirement with “conspicuous” signage to notify patients of their right to a copy of their contact lens prescriptions. The bill also proposes banning automated prescription verification calls.
REACTIONS TO THE FINAL RULE
Practitioners interviewed for this article were unanimously concerned with the administrative and financial burden to comply with the Final Rule. Specifically, some cannot afford or are not permitted to hire additional personnel to manage the extra paperwork, which can cost thousands of dollars in labor to scan the signed slips into the electronic medical record (EMR). Furthermore, practices that use a patient portal are already offering patients access to their contact lens prescriptions, which they can download at any time. Some patients have been confused as to why they have to sign the additional form. Finally, the initial implementation on Oct. 16, 2020 (prior to the delay) negatively impacted practitioners already reeling from new office protocols related to COVID-19.
Alysa Bernstein, JD, of the FTC responded in an e-mail on how the Final Rule has been received by eyecare providers, online contact lens retailers, and consumers: “The reception to the rule amendments has been both positive and negative, with eyecare providers and retailers praising some aspects of the amended rule while being less happy with other parts. Retailers, for instance, have praised our efforts to ensure that consumers receive a copy of their prescriptions but were less pleased with our new requirement that they record automated verification messages. Eyecare providers, on the other hand, should benefit from better verification messages but have indicated that obtaining confirmation of prescription release creates burden for them, especially during the COVID pandemic. As far as the consumers—who of course, are the true focus of the rule—we have not had much feedback at all. But we firmly believe that they will benefit from greater access to their prescriptions and from more reliability and accuracy when they get their prescription verified by contact lens retailers.”
Bernstein noted that the FTC staff is always looking out for changes in the industry and issues that it may need to address, but currently, “…staff is focused on ensuring that prescribers and sellers are aware of their obligations under the recent amendments to the Contact Lens Rule.”
Knowing that the FTC desires to review the viability of substituting contact lenses and lengthening prescription expiry reminds all eyecare professionals and industry stakeholders of how important it is to continue educating regulatory decision makers about our industry.
QUESTIONS REMAIN FOR SPECIALTY CONTACT LENSES
While the FCLCA and the Final Rule target the broad market of mass-produced contact lenses, it has also included specialty contact lenses by association. Many specialty contact lens prescribers are confused by how the regulations apply to patients who wear medically necessary contact lenses for eye diseases.
According to Bernstein of the FTC, custom-designed lenses were addressed in the original Rule’s statement of basis and purpose. She emphasized that the FCLCA, the Act that directed the FTC to promulgate the Contact Lens Rule, does not permit the Commission by rule to grant an exception to the release requirement for custom-designed soft and GP lenses. “Moreover, the record indicates that some sellers (other than prescribers) can supply such lenses to consumers. Consequently, the creation of an exception to the release requirement for custom-designed soft and rigid gas permeable lenses would be inconsistent with the Act’s goal of meaningful prescription portability and increased consumer choice. The Final Rule accordingly includes no such exception,” she said.
Still, the approach by the FTC does not acknowledge the intricacies critical to the specialty lens market. Christine Sindt, OD, director of Contact Lens Service at the University of Iowa and president of EyePrint Prosthetics, expressed, “My concern with customized contact lenses is this—what constitutes a prescription?” She indicated that for individually customized lenses, the usual defining lens characteristics of base curve, diameter, and power are simply not enough. The new-generation custom lenses will not even have base curves and cannot be verified. Dr. Sindt asked, “Do you release the cut file of the lens (example available below) and invoice numbers, which are meaningless to another manufacturer?” Furthermore, she said, “For the really sick, diseased eyes, who is responsible for the eyeball underneath of the lens? If a patient gets an online company to make the lens with just base curve, power, and diameter, the patient won’t get the same lens prescribed.” Dr. Sindt believes that the Rule was put in place by people who have good intentions but don’t understand the entire specialty prescribing process.

Dr. Sindt indicated that with regard to contact lenses for most eye disease applications, the choice for patients often is not where to get their contact lenses, but which practitioner can help them with their complex ocular health issue. “If the FTC wants to regulate specialty lenses, the better way to do it is to notify patients that the opportunity to choose is when they select their practitioner. The FTC seems to believe that all contact lenses are products that consumers can buy wherever they choose. Yet, this understanding does not apply to these custom contact lenses,” she said.
Indeed, a disproportionate number of patients who have keratoconus, corneal transplants, and other corneal irregularities require treatment with proprietary, custom contact lens designs, which are inherently not shoppable by patients. The difficult conundrum is that—despite the desire by many specialty contact lens practitioners to comply with the Final Rule and The Act—they are concerned about the absence of provisions to ensure that patients who have diseased eyes receive lenses with the critical proprietary specifications required for safe and effective outcomes. If a patient receives a lens from an outside source that matches base curve, material, diameter, and power—but without the needed sagittal depth or customized peripheral curves (Figure 1)—the patient may be predisposed to corneal abrasion or even transplant rejection. Practitioners in this situation have a fiduciary responsibility to put the interests of their patients ahead of themselves. However, releasing a contact lens prescription in these situations can put patients at great risk.
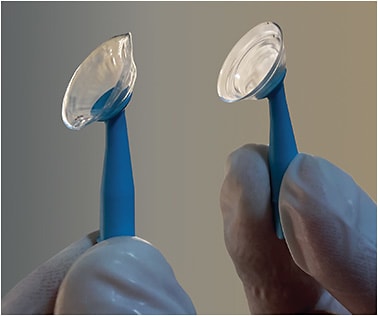
Asked about the customized contact lens designs for medical eye disease that only specific laboratories can produce, FTC attorney Bernstein said, “To staff’s knowledge, this issue was not one that was raised in thousands of comments received by the FTC in its recent Rule review, and we have not had practitioners contact us about this.” When asked about post-graft and keratoconus patients potentially receiving contact lenses that do not meet proprietary specifications, Bernstein reiterated that the Act is clear that there is no exception for these special contact lenses, adding, “I would hope that the prescriber could, in these instances, convince the patient that, for medical reasons, the patient should not try to substitute other lenses. At that point, it is up to the patient.”
Practitioner concern also exists regarding the compatibility of the Final Rule with future contact lenses that move beyond traditional vision correction. Novel designs in the pipeline include contact lenses with accommodating optics, drug delivery, augmented reality, and health-sensing capabilities. For such individualized lenses, a “prescription” may resemble more of a receipt showing an invoice or product serial number. To be sure, there is room for stakeholders in the specialty contact lens industry to share with the FTC how the Final Rule can better serve patients who have medical eye disease and those needing custom contact lenses while also working together to minimize administrative burden.
UNIVERSAL PATIENT PORTAL: AN EFFICIENT AND SYSTEMIC SOLUTION
Aaron See, senior vice president of Manufacturer Partnerships at ABB Optical Group, noted that the Final Rule adds additional burden on practitioners and staff. It consumes resources and makes practices less efficient. It is another burden from a record-keeping standpoint for the independent practices. “Practitioners will look at areas to increase efficiency with patient flow, value per patient, and pricing strategies. No question, acknowledgement of prescription release will be captured digitally and housed where practitioners are already placing that patient information: in their EMR,” he said.
This is where EMRs can help, particularly if there is robust functionality. The workflow is similar to collecting a patient’s acknowledgement of receipt of the Notice of Privacy Practices (NPP). However, the contact lens prescription acknowledgement has a greater and recurring burden, as it must be collected each time a patient receives a new contact lens prescription.
Jason Rohrer, product manager with Eye Care Leaders, an eyecare-specific EMR provider, said, “Regulatory changes are unavoidable. So, we work hard to incorporate required regulatory elements into existing product workflows to minimize the providers’ burden as much as possible.” He explained that their teams are diligently tackling this right now in an effort to ease the burden of this new FTC regulation. How long will it take to bring to the market new software to ease the burden? Rohrer would not offer exact timing, but he said that, “The time required to research, design, build, test, and rollout new regulatory changes into a product depends on two things: the requirement and the product.”
When asked whether the FTC can provide resources for EMR software developers, Bernstein of the FTC said, “We have had no software developers contact us about developing functionality to comply with the amendments to the Final Rule. If they do contact us, we would be happy to discuss the requirements of the Rule. If practitioners are waiting for the development of such systems, in the interim, they can avail themselves of the other alternatives to comply with the Rule.” The alternatives that she alluded to include providing a digital copy or having patients sign the prescription, the examination sales receipt, or a separate form.6 Rohrer of Eye Care Leaders cautioned that, “…while an attorney or the government regulatory body can offer guidance, neither can provide a guarantee to the software provider or their customers that a given solution will always pass a future audit.”
THE IMPACT OF THE FINAL RULE CONFOUNDED BY COVID-19
So far, it is difficult to assess the effect of the Final Rule on contact lens sales. In part, insufficient time has passed to evaluate changes in the channel sales of contact lenses. Furthermore, COVID-19 has accelerated online purchasing of contact lenses, which confounds the contribution of the Final Rule. Many patients will not go back to buying contact lenses in person. Because patients desire convenience, COVID-19 is leading eyecare professionals to adapt and implement improved processes and services. This includes using e-commerce platforms and manufacturer subscription services with direct shipping. Patients may still want to purchase contact lenses through their prescriber, but they want the added convenience.
THE NEW ECOLOGY OF CONTACT LENS PRESCRIBING
With increasing consumerism, the prospect of mass-produced contact lenses becoming deregulated—that is, purchasable without a prescription—in the United States is worth contemplating. Since 1976, the FDA has regulated daily wear contact lenses as class II (moderate risk) medical devices and continuous wear contact lenses as class III (high risk) medical devices. This means that contact lenses require professional oversight. But today, like it or not, future deregulation of contact lenses is a possibility, encouraged by online contact lens company interests. De jure deregulation occurs if regulatory bodies decide that substitution, generics, lax expiries, and self-verification apply to contact lenses. De facto deregulation occurs due to globalization of business and technological advances, with inadequate regulatory enforcement or with authorities looking the other way.
For a preview of what the U.S. contact lens market may look like in the years ahead, we can peer into the European Union, where contact lenses are completely deregulated in 25 of the 27 countries. In other words, a consumer can purchase a contact lens without a prescription in most of Europe. According to Edward Dean Butler, the founder of LensCrafters who has lived in the United Kingdom for 33 years, “De facto deregulation is headed to the U.S. contact lens market, and it will be impossible to stop. A few major online non-U.S. vendors are likely to soon enter the U.S. market, shipping from outside the country. It is highly unlikely that state legislation can stop this, and certainly federal legislation will not do so.” Additionally, he noted, accurate, reliable online acuity testing at home is available, but it will soon vastly improve. “The prototypes exist. I have seen them. When this happens, the current U.S. system will start to fall like a house of cards,” he said.
According to Butler, deregulation in Europe started in Scandinavia two decades ago, and it led to a significant increase in contact lens sales, going from about 10% of corrective lenses sold to now about 30%. “The contact lens manufacturers are unlikely to tell you, but they love deregulation,” he said. In Europe, contact lens prescriptions are self-certified. “Even in the United Kingdom, where verification is still required, authorities do not challenge this. No nation in Europe does,” said Butler. Following contact lens deregulation in Europe, Butler said, “The incidence of corneal problems decreased because patient compliance improved. Keep in mind that Europe is mostly a daily [disposable] lens market. There is less tendency for consumers to extend lens use of daily disposables. I am told on good authority that there are at least two studies showing this, but they have never been released because the manufacturers do not want to risk creating professional relations problems in other markets.”
While a contact lens vending machine (Figure 2) is an unusual sight to most U.S. practitioners, Butler said that, “Vending machines work, and they are widely used in Russia, Belarus, and parts of Germany. Inventory in each vending machine is adjusted to what customers from that area desire. The very latest machines connect in real time with a consultant.” He continued, “Customers are primarily younger women who are willing to pay premium prices for the convenience of purchasing one or two boxes at a time.” Butler said that the best locations tend to be gas stations, but grocery stores, especially the hypermarket format (i.e., a big-box store that combines a supermarket with a department store), also work.

Arguably, de facto deregulation already exists to some degree for U.S. consumers. Major online sellers allow U.S. consumers to browse pricing as well as ratings and reviews for various contact lens brands as if they represent common consumer goods rather than prescriptive medical devices.
Some major online contact lens sellers offer online contact lens prescription renewal, minimizing the public perception that contact lenses require professional oversight. Jeffrey Sonsino, OD, past chair of the Contact Lens & Cornea Section of the AOA and member of the Health Care Alliance for Patient Safety, said, “Online resellers are exhibiting poor behavior and need to be reined in by the FTC. They are spreading messages on social media such as ‘skip the trip’ and ‘no one wants an air puff’ that are dangerous for public health.”
However, it appears that online contact lens prescription renewal is here to stay. Ophthalmologist Saya Nagori, MD—who in 2015 co-founded Simple Contacts, the online contact lens prescription renewal service—shared that, “The writing is on the wall for the future of contact lens prescribing. As a clinician, you cannot be fearful. There was negativity and bullying directed to me when I pioneered online prescription renewal. Now, all of the online companies are doing this.” She noted that it took the COVID-19 lockdown for most eyecare practitioners to embrace telemedicine and online prescription renewal. When renewing what a patient already wears, Dr. Nagori continued that, “An asymptomatic patient who has 20/20 vision and no co-morbidities has an extremely low likelihood of having ocular pathology. Most corneal pathologies such as a corneal ulcer or abrasion are symptomatic. For healthy patients who do not have any noteworthy medical or ocular history, telemedicine is a valuable adjunctive resource for patients and for the overall healthcare system.”
LIGHTING A PATH FORWARD
Today, virtually all U.S. consumers have a camera readily available in their smartphone. Yet, there is an enduring role for professional photographers, particularly those who have dedicated, high-quality equipment and an understanding of lighting and composition to bring value to their customers. In parallel fashion, even with contact lens deregulation, whether de jure or de facto, contact lens professionals will continue to have a meaningful role. Increased contact lens purchases by consumers could grow the demand for professional services to optimize outcomes. Self-verification of contact lens prescriptions can only go so far, such that practitioners in a deregulated environment would see more cases requiring a higher degree of skill and in-person service. This is where myopia management, presbyopia treatment with contact lenses, and scleral contact lens prescribing provide great opportunities. Aaron See noted, “Specialty contact lens prescribing is the epitome of practitioner engagement with patients.”
NO LIMIT ON CONTACT LENS QUANTITY
Eyecare professionals are concerned that the Final Rule condones the purchase of much more than an annual supply, unlike limits on prescription medication. For example, one online contact lens retailer allows a user to order 24 boxes of a legacy two-week lens for each eye, which is a 6-year supply. This can dissuade routine eye health examinations.
Alysa Bernstein, JD, of the Federal Trade Commission, addressed this concern as follows: “The Commission determined not to impose such a quantity limit in its recent Rule review. First, staff received no compelling evidence of a problem involving consumers purchasing large quantities of contact lenses.”
Furthermore, she indicated that the Commission expressed its view that rather than increasing patient eye health and safety, [quantity limit restrictions] could have the opposite effect. For example, if a consumer is running out of contact lenses and does not have time to see a prescriber promptly, there is a significant chance that the consumer will not adhere to the recommended contact lens replacement schedule and will instead try to ‘‘stretch out’’ the remaining lenses by re-wearing them until he or she can visit a prescriber. “The failure to replace lenses is a well-documented cause of many contact lens-related health issues. Absent empirical evidence that a substantial number of consumers are obtaining excessive amounts of contact lenses, or are not returning to their prescribers for eye examinations, the Commission believes that the risk of not replacing lenses outweighs the harm of consumers obtaining more lenses than strictly anticipated by the length of a contact lens prescription,” she said. Ms. Bernstein continued, “Lastly, it appears that the majority of patients see their eyecare prescriber annually; thus, they are likely getting new prescriptions, and any contact lens wear issues should be addressed at the visit.”
Meanwhile, Michele Andrews, OD, vice president, Professional & Government Affairs, Americas at CooperVision, said that, “As a global organization with a presence in other countries that have fewer regulations, our international experience informs us that the practitioner is critical to the overall patient success. In general, dropout rates in unregulated countries are double or higher than in the United States. The role of the ECP is critical in lens selection and in working with patients and making sure that they have good habits. We feel that the FTC acknowledges this.” Other large manufacturers were also invited to comment but did not respond or declined.
For the mass-produced contact lens category, one strategy to provide greater patient convenience is to offer contact lens subscriptions with direct delivery. Butler said, “U.S. ECPs are their own worst enemies, because many encourage patients to buy an annual supply, and they limit the prescription to one year. I have seen research indicating that the average independent ECP loses 80% of potential replacement contact lens sales for these reasons.” According to Butler, manufacturer subscription programs can win back half of lost business if ECPs accept selling lenses quarterly or even monthly.
No doubt, online companies will continue trying to move patients and their lens purchases out of eyecare practices with convenient virtual offerings. When asked what ECPs can do about this, Dr. Nagori said, “Recognize that well-informed patients are a good thing. Being informed empowers them to advocate for and to improve their care. They understand the importance of in-person care and when telemedicine is useful. By emphasizing your experience, training, and expertise, encourage patients to see you in the office when that is appropriate. Provide them with options and convenience, and help them navigate their vision plan. If you offer competitive pricing and take the burden off of patients, that enhances the patient experience.”
In the wake of regulatory trade decisions, the U.S. mass-produced contact lens market careens toward a European-like model. U.S. prescribers forecast negative effects. However, it is relevant to understand “impact bias”: in the psychology of affective forecasting, there is a tendency to overestimate the magnitude and duration of how you will feel after specific events. This suggests that in a deregulated environment, prescribers will not feel as bad nor for as long as they might believe. In other words, life will go on.
Regardless of the course that our contact lens market takes, U.S. practitioners can take solace in knowing that their patients will continue seeking their services, just as patients continue to seek contact lens professionals in Europe. Furthermore, the expansion of contact lens technology—with scleral lenses, myopia management, drug delivery, augmented reality, and other novel applications—will drive new patient-practitioner relationships, even as online contact lens sellers marginalize and co-opt existing patient-practitioner relationships. CLS
REFERENCES
- Federal Trade Commission. The Contact Lens Rule. Fall 2015. Available at https://www.reginfo.gov/public/do/eAgendaViewRule?pubId=201510&RIN=3084-AB36#:~:text=As%20part%20of%20its%20ongoing%20systematic%20review%20of,of%20any%20technological,%20economic,%20or%20other%20industry%20changes . Accessed April 7, 2021.
- Federal Trade Commission. FTC Announces Final Amendments to the Agency’s Contact Lens Rule. 2020 Jun 23. Available at https://www.ftc.gov/news-events/press-releases/2020/06/ftc-announces-final-amendments-agencys-contact-lens-rule#:~:text=The%20Contact%20Lens%20Rule%20already%20prohibits%20prescription%20alteration%2C,manufacturer%20other%20than%20that%20prescribed%20to%20the%20consumer . Accessed April 7, 2021.
- Health Care Alliance for Patient Safety. Patient Safety Advocates Applauds Congressional Directive to Delay Implementation of the FTC Contact Lens Rule. 2020 Dec 28. Available at https://www.patientsafetytoday.com/aps_applauds_congressional_directive_to_delay_implementation_of_the_ftc_contact_lens_rule . Accessed April 7, 2021.
- Statement of Commissioner Rebecca Kelly Slaughter in the Matter of Contact Lens Rule Review. Commission File No. R511966. June 23, 2020. Available at https://www.ftc.gov/system/files/documents/public_statements/1577127/r511995_contact_lens_final_rule_-_rks_concurrence.pdf . Accessed April 7, 2021.
- American Optometric Association. Contact Lens Rule Modernization Act Introduced in the U.S. 2020 Sep 16. Available at https://www.aoa.org/news/advocacy/federal-advocacy/contact-lens-rule-modernization-act-introduced-in-the-us-senate?sso=y . Accessed April 7, 2021.
- American Optometric Association. Contact Lens Rule Compliance Toolkit. 2020 Oct. Available at https://www.aoa.org/AOA/Documents/doctor resources/Contact-Lens-Rule-Compliance-Toolkit.pdf/ . Accessed April 7, 2021.




