
Topical eye drops are the most common method of delivering drugs to manage ocular disease; however, there are many limitations to this widely accepted method. Eye drops have low bioavailability (< 5%) due to high tear turnover rates, blinking, nasolacrimal drainage, nonproductive absorption by the conjunctiva, and low permeability of the cornea (Alvarez-Lorenzo et al, 2019; Urtti, 2006; Zhang et al, 2020).
Using soft contact lenses (CLs) to deliver ophthalmic medication to the ocular surface has been a promising method to overcome these limitations. Soft CLs split the tear film into a pre- and post-lens tear film. The post-lens tear film is very thin, with a relatively low turnover rate (Nichols and King-Smith, 2004), which promotes an increase in concentration of ophthalmic drugs behind the lens and increases ocular penetration (Alvarez-Lorenzo et al, 2019; Li and Chauhan, 2006).
Using CLs as an ocular drug delivery method can decrease the frequency of drug administration, minimize systemic absorption, improve treatment compliance, remove the need for preservatives, and provide a more controlled drug release profile (Alvarez-Lorenzo et al, 2019; Winfield et al, 1990).
Challenges of Contact Lens Drug Delivery
Using CLs for drug delivery is not without its challenges. Here are a few to consider:
- For a CL to deliver drugs to the eye in therapeutically relevant amounts, the chemical compatibility of the drug and lens material must be optimized. A drug with a strong affinity for the CL material may increase drug uptake but result in an unacceptably prolonged drug release (Zhang et al, 2020).
- The manufacturing method must ensure that the drug is still viable post CL production. There are two main manufacturing concepts: the drug is incorporated into the CL monomer mix prior to CL curing and extraction, or the drug is added after the CL is fully polymerized and hydrated. Upon sterilization of the CL material, the chemical nature of the drug, as well as the drug concentration and degradation rate, may change. As different CL powers have different lens volumes, each CL may also need to be tailored to the drug properties, the CL material, and the lens power—a costly and timely process.
- Like all medical devices, drug delivery CLs must demonstrate safety and efficacy through pharmaceutical and device reviews (Zaki et al, 2019). Another factor to consider is CL replacement modality. For chronic diseases, an overnight or monthly replacement CL may be the preferential modality. Daily wear lenses must be able to withstand the daily cleaning and soaking steps (Zhu et al, 2011). Daily disposable lenses can avoid this issue, but the manufacturing process would need to be scaled up to ensure a sufficient number of lenses are produced.
- Regardless of the CL replacement modality, all packaged drug-releasing CLs must demonstrate long-term stability with minimal drug degradation and consistent drug concentration (Maulvi et al, 2017). The CLs must withstand manufacturing, packaging, shipping, and storage in packaging solution before being placed on the eye.
Ocular Drug-Delivering Technologies
In various disease states, therapeutic “bandage lenses” are commonly used in conjunction with antibiotics and steroids (Baenninger et al, 2014; Jacobs et al, 2021; Karlgard et al, 2004; Miller et al, 2019). However, beyond this common practice, few have studied the impact of the concurrent use of pharmaceutical agents and commercial CLs.
Many major ophthalmic medications have been studied in vitro for uptake and release profiles (Dixon and Chauhan, 2017; Karlgard et al, 2003; Solui et al, 2012; Jones et al, 2021). Generally, the trend for drug-releasing CLs demonstrates significant amounts of drug uptake and release in vitro, with the release profiles showing an uncontrolled burst release over the first few minutes of wear and poor delivery over extended periods of time (Solui et al, 2012; Jones et al, 2021; Hui et al, 2008).
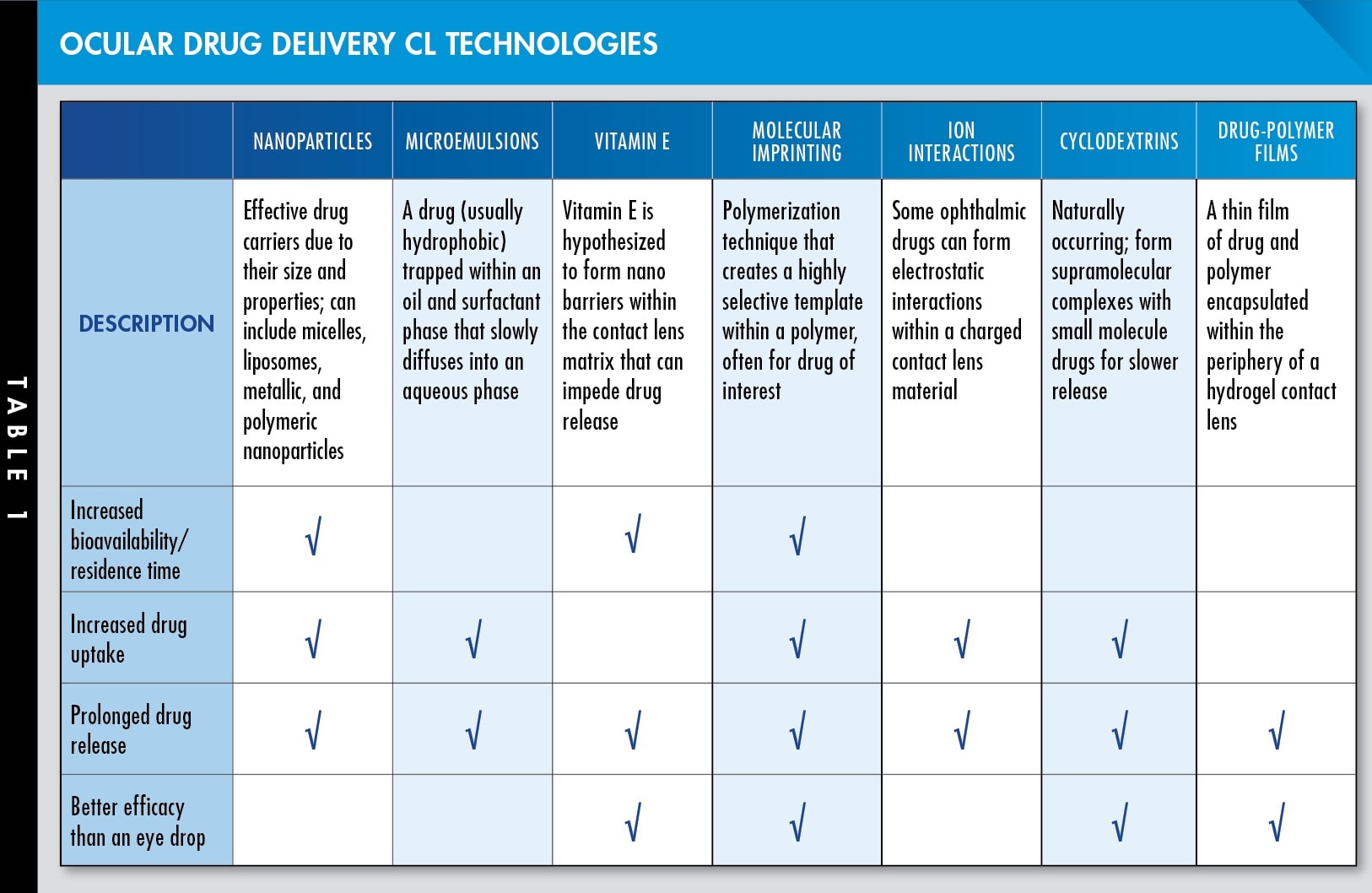
As a result, there are many ongoing studies to find ways to overcome these limitations and bring ocular drug delivery CLs one step closer to being clinically relevant products. Many of these technologies are summarized in Table 1, viewable in the CLS digital edition. CLS
References
- Alvarez-Lorenzo C, Anguiano-Igea S, Varela- García A, Vivero-Lopez M, Concheiro A. Bioinspired hydrogels for drug-eluting contact lenses. Acta Biomater. 2019 Jan;84:49-62.
- Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006 Nov 15;58: 1131-1135.
- Zhang X, Cao X, Qi P. Therapeutic contact lenses for ophthalmic drug delivery: major challenges. J Biomater Sci Polym Ed. 2020 Mar;31:549-560.
- Nichols JJ, King-Smith PE. The impact of hydrogel lens settling on the thickness of the tears and contact lens. Invest Ophthalmol Vis Sci. 2004 Aug;45:2549-2554.
- Li C-C, Chauhan A. Modeling Ophthalmic Drug Delivery by Soaked Contact Lenses. Ind Eng Chem Res. 2006 Apr;45:3718-3734.
- Winfield AJ, Jessiman D, Williams A, Esakowitz L. A study of the causes of non-compliance by patients prescribed eyedrops. Br J Ophthalmol. 1990 Aug;74:477-480.
- Zaki M, Pardo J, Carracedo G. A review of international medical device regulations: Contact lenses and lens care solutions. Cont Lens Anterior Eye. 2019 Apr;42:136-146.
- Zhu H, Bandara MB, Vijay AK, Masoudi S, Wu D, Willcox MDP. Importance of rub and rinse in use of multipurpose contact lens solution. Optom Vis Sci. 2011 Aug;88:967-972.
- Maulvi FA, Choksi HH, Desai AR, et al. pH triggered controlled drug delivery from contact lenses: Addressing the challenges of drug leaching during sterilization and storage. Colloids Surf B Biointerfaces. 2017 Sep 1;157:72-82.
- Baenninger P, Dinah C, Figueiredo FC. Survey on bandage contact lens practice in the United Kingdom. J Clin Exp Ophthalmol. 2014;5:1-9.
- Jacobs DS, Carrasquillo KG, Cottrell PD, et al.: CLEAR - Medical use of contact lenses. Cont Lens Anterior Eye. 2021 Apr;44:289-329.
- Karlgard CC, Jones LW, Moresoli C. Survey of bandage lens use in North America, October-December 2002. Eye Contact Lens. 2004 Jan;30:25-30.
- Miller DD, Hasan SA, Simmons NL, Stewart MW. Recurrent corneal erosion: a comprehensive review. Clin Ophthalmol. 2019 Feb 11;13:325-335.
- Dixon P, Chauhan A. Effect of the surface layer on drug release from delefilcon-A (Dailies Total1((R))) contact lenses. Int J Pharm. 2017 Aug 30;529:89-101.
- Karlgard CC, Wong NS, Jones LW, Moresoli C. In vitro uptake and release studies of ocular pharmaceutical agents by silicon-containing and p-HEMA hydrogel contact lens materials. Int J Pharm. 2003 May 12;257:141-151.
- Soluri A, Hui A, Jones L. Delivery of ketotifen fumarate by commercial contact lens materials. Optom Vis Sci. 2012 Aug;89:1140-1149.
- Jones L, Hui A, Phan C-M, et al. CLEAR - Contact lens technologies of the future. Cont Lens Anterior Eye. 2021 Apr;44:398-430.
- Hui A, Boone A, Jones L. Uptake and release of ciprofloxacin-HCl from conventional and silicone hydrogel contact lens materials. Eye Contact Lens. 2008 Sep;34:266-271.



