
LEARNING METHOD AND MEDIUM
This educational activity consists of a written article and 20 study questions. The participant should, in order, read the Activity Description listed at the beginning of this activity, read the material, answer all questions in the post test, and then complete the Activity Evaluation/Credit Request form. To receive credit for this activity, please follow the instructions provided below in the section titled To Obtain CE Credit. This educational activity should take a maximum of two hours to complete.
CONTENT SOURCE
This continuing education (CE) activity captures key statistics and insights from contributing faculty.
ACTIVITY DESCRIPTION
The goal of this article is to review the most recent disinfection guidelines for multi-patient-use diagnostic lenses so practitioners can apply this knowledge to their clinical practice and contact lens instructors can incorporate it in an academic setting.
TARGET AUDIENCE
This educational activity is intended for optometrists, contact lens specialists, and other eyecare professionals.
ACCREDITATION DESIGNATION STATEMENT
This course is COPE-approved for two hours of CE credit.COPE Course ID: 80717-CL
DISCLOSURES
Pam Satjawatcharaphong, OD, reports no conflicts of interest.Lindsay Sicks, OD, has received remuneration from Alcon Laboratories and RevHealth.
DISCLOSURE ATTESTATION
The contributing faculty member has attested to the following:
- That the relationships/affiliations noted will not bias or otherwise influence their involvement in this activity;
- That practice recommendations given relevant to the companies with whom they have relationships/affiliations will be supported by the best available evidence or, absent evidence, will be consistent with generally accepted medical practice;
- That all reasonable clinical alternatives will be discussed when making practice recommendations.
TO OBTAIN CE CREDIT
To obtain COPE CE credit and instant certificate processing for this activity, read the material in its entirety and consult referenced sources as necessary. Please take the post test and evaluation by following the link below and clicking on the CE Information tab.
Specialty Contact Lens Disinfection
Upon passing the test, you will immediately receive a printable PDF version of your course certificate for COPE credit. On the last day of the month, all course results will be forwarded to ARBO with your OE tracker number, and your records will be updated. You must score 70% or higher to receive credit for this activity. Please make sure that you take the online post test and complete the evaluation on a device that has printing capabilities.
NO-FEE CONTINUING EDUCATION
There are no fees for participating in and receiving credit for this CE activity.
DISCLAIMER
The views and opinions expressed in this educational activity are those of the faculty and do not necessarily represent the views of Contact Lens Spectrum. This activity is copyrighted to PentaVision LLC Powered by BroadcastMed ©2022. All rights reserved.
This activity is supported by unrestricted educational grants from ABB Optical, Acculens, Art Optical, Boston Sight, and Contamac.
CE Questions? Contact CE@pentavisionmedia.com for help.
RELEASE DATE: NOVEMBER 1, 2022EXPIRATION DATE: SEPTEMBER 21, 2025
The relationship between patient noncompliance with reusable contact lens instructions, poor case hygiene, and adverse ocular events has been well documented. Complications range widely, from minor irritation and vision reduction to severe, sight-threatening infections.
Although globally there is growth in the prescription of daily disposable contact lenses, a 2019 survey of new fits worldwide shows that just over one-half of soft contact lenses are reusable.1 Primary eye-care providers possess the expertise to fit and prescribe contact lenses, but also have a responsibility to educate patients regarding the proper care and disinfection of contact lenses. Unfortunately, although patients are educated about potential risks of poor lens care and case hygiene, studies show a disconnect between risk awareness and actual compliance.2
If they are noncompliant, contact lens wearers are at risk for infection by many microbes in the course of lens wear. Fortunately, most contact lens-related adverse events linked to poor compliance are either self-limiting or easily treated, and a majority resolve without long-term sequelae. However, more severe complications of contact lens wear, such as microbial keratitis (MK), can potentially cause significant vision loss.3 The risk of MK increases if patients sleep in lenses or expose their lenses and cases to nonsterile water.4-6
One complication associated with exposure to nonsterile water (e.g., wearing lenses while showering or swimming) is Acanthamoeba keratitis (AK). The protozoan Acanthamoeba can affect soft contact lens and GP contact lens wearers. Unfortunately, it is notorious for being misdiagnosed in its early stages due to mild or nonspecific clinical signs (Figures 1 and 2). Practitioners are often more familiar with the classic late sign of infection—a ring infiltrate (Figure 3)—but once the disease has reached this stage, treatment is much more challenging and the prognosis is poor.

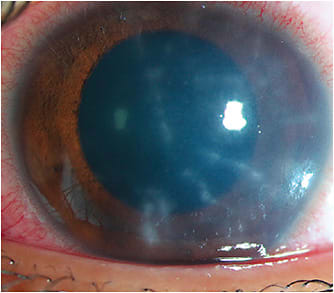

Since AK is often difficult to diagnose in its early stages but is also sight-threatening if treatment is delayed, stopping all potential lens and case exposure to nonsterile water is critical to reducing the risk of infection. If Acanthamoeba infection is suspected, the diagnosis is confirmed through tissue culture or visualization of cysts with in vivo confocal microscopy (Figure 4). Due to the low sensitivity of Acanthamoeba cultures (reported at 54% to 68%), and with results taking at least one week to receive, the advantages conferred by confocal microscopy are evident.7 This quick and effective tool has superior efficacy, is noninvasive, and has a positivity rate as high as 94.6%.7

However, access to confocal diagnostic imaging often requires referral to a corneal specialist or large academic medical center with the necessary instrumentation. Therefore, it is advisable to know which practitioners in your local area can quickly provide this service if you suspect one of your patients has AK.
Acanthamoeba infection is influenced by the quality of the domestic water supply, which is affected by disinfection protocol, supply route, and water hardness.5 We routinely educate our contact lens patients regarding proper lens care procedures, including avoiding tap water exposure; however, less attention is devoted to the potential for adverse events linked to improper cleaning and disinfection of the multi-patient-use soft and GP contact lenses used for diagnostic fitting in both clinical and academic settings.
OVERVIEW OF 2018 ISO STANDARD AND 2020 TECHNICAL REPORT
In March 2018, the International Organization for Standardization (ISO) released item “19979:2018(E) Ophthalmic optics - Contact lenses - Hygienic management of multi-patient-use trial contact lenses” (the 2018 ISO Standard).8 Outlined in the 2018 ISO Standard are detailed processes for multi-patient-use diagnostic contact lens disinfection during and after the in-office contact lens fitting process.
In 2019, the American Academy of Optometry (AAO) Cornea, Contact Lenses and Refractive Technologies Section and the American Optometric Association (AOA) Contact Lens & Cornea Section collaborated to create a clinically relevant set of guidelines for practitioners to use in the disinfection process. This flowchart, titled “Guidelines for Handling of Multi-Patient Contact Lenses in the Clinical Setting,” was published in 2020.9
This work has ushered in a new gold standard for lens disinfection during a global pandemic and heightened awareness of the potential for transmission of infectious diseases. The COVID-19 pandemic has strengthened the understanding of the importance of in-office disinfection processes. In addition, a recent publication confirms the disinfection process outlined in the 2018 ISO Standard and the guidelines should protect against any direct transmission of SARS-CoV-2 from multi-patient-use lenses.10
The guidelines aim to present a standardized, easy-to-follow, and practical protocol to implement the 2018 ISO Standard into a clinic’s workflow while minimizing the risk of pathogen transfer between patients when working with multi-patient-use diagnostic contact lenses. The guidelines do not match the 2018 ISO Standard verbatim; instead, they represent a practical interpretation of the guidance provided within the text of the standard. Our further review and understanding of both documents have resulted in a workflow (outlined below) that serves well in both academic and clinical settings.
The 2018 ISO Standard and the guidelines both specify the same three categories of multi-patient-use diagnostic contact lenses: GP, soft, and hybrid.8,9 The GP lens category includes any GP lens design: corneal, limbal, or scleral. The disinfection process for all rigid lenses is the same regardless of material type (e.g., polymethyl-methacrylate [PMMA] lenses follow the same procedure as more contemporary fluoro-silicone acrylate and silicone acrylate lens materials). The soft lens category includes both conventional hydrogel and newer silicone hydrogel lens materials. Finally, the hybrid (also known as composite) lens category is for those lenses that contain a GP lens center bonded to an outer soft lens skirt.8
Guidelines: Initial Steps for All Lens Materials The 2018 ISO Standard suggests that each used diagnostic lens be rubbed with a compatible daily surfactant cleaner, according to manufacturer instructions, before disinfection in hydrogen peroxide solution.8 This cleaning step removes any attached deposits or debris from the lens surface, ensuring that proper disinfection can occur afterward. After cleaning, but prior to disinfection, the 2018 ISO Standard then suggests using a multipurpose solution (MPS) or saline to rinse off the daily surfactant cleaner.
Avoid using tap water for rinsing, as this introduces the risk of Acanthamoeba infection.9 Following rinsing, initate diagnostic lens disinfection with commercially available 3% ophthalmic-grade hydrogen peroxide contact lens solution. Consider placing a paper towel or single-use dental bib on the bench or tabletop to maintain an aseptic field during lens disinfection and to catch any spills.
Place the contact lens inside the well of a non-neutralizing lens case (such as a flip-top or screw-cap style disposable case) or another appropriate container (we prefer a silicone ice cube tray, as subsequently outlined). Fill the well with a commercially available 3% ophthalmic grade hydrogen peroxide solution approved for use with contact lenses. Ensure there is sufficient solution to cover the lens completely. Leave the lens to soak for three hours.
After the soak, a practitioner should treat the lens as sterile; that is, wash hands and don gloves prior to handling it from this step forward. Remove the lens using a sterile cotton-tipped applicator or disinfected contact lens tweezers, if needed, and follow the remaining steps as directed, based on the type of lens being disinfected.
Guidelines: Next Steps for GP Lens Materials Rinse the lens thoroughly with either saline or an MPS. Dry the lens completely with a lint-free tissue such as a delicate task wipe. Store the disinfected lens dry in a disinfected case.
Guidelines: Next Steps for Soft and Hybrid Lenses Fill a neutralizing lens case to the fill line with commercially available 3% hydrogen peroxide solution approved for use with contact lenses. Transfer the disinfected lens to the neutralizing case. Place the lens holder in the case, tighten the cap, and soak for at least six hours (or add neutralizing tablet and store according to the solution manufacturer). Once neutralization is complete, wash your hands and apply gloves.
Remove the lens from the case using a sterile cotton-tipped applicator or disinfected contact lens tweezers, if needed. Rinse thoroughly with saline or a compatible MPS and store in a disinfected case or vial filled with a compatible MPS. Confirm that the solution you are using is compatible with the lens type you are disinfecting. For example, some soft lens solutions are recommended for use with hybrid lenses, while others are not compatible. If you are unsure whether a solution is compatible, consult the package insert, check the manufacturer’s website, or discuss it with a member of the laboratory’s consultation team before use.
DISINFECTING LENS CASES
When possible, avoid contaminating the original storage case for each diagnostic lens. Transfer the lens(es) to be fit into a separate disposable case before application. Disposable flip-top or screw-cap style cases are useful for this process. Once the fitting has been completed, remove the lens and place it back into the disposable case. Then follow the disinfection procedure for the lens type, as previously outlined.
Disinfect the original diagnostic lens case or vial by filling it with commercially available 3% ophthalmic-grade hydrogen peroxide solution. Remove the rubber stopper from any lens vial that contains one. The stopper and vial should both soak in 3% ophthalmic grade hydrogen peroxide for three hours for complete disinfection.
Once the vial and stopper have been disinfected, handle them with gloves. Discard the hydrogen peroxide solution and rinse the original lens vial or case with fresh saline or an MPS. Fill the vial or case with solution and shake for 15 to 30 seconds before discarding the solution. Repeat two more times to ensure the complete removal of all traces of hydrogen peroxide.
For GP lenses, dry the case thoroughly with a lint-free tissue prior to adding the dry lens for storage. For soft and hybrid lenses, fill the case with a compatible solution prior to adding the lens. Discard the disposable case.
RECORD AND EXPIRATION OF DISINFECTION
Each practice should maintain a diagnostic lens disinfection log. The log should include a patient reference (such as the medical record number), lens manufacturer and brand/type, lens parameters (base curve, power, diameter, color, and any other identifying information such as vault, skirt, or sagittal height), date of lens use, date of disinfection, date of expiration of disinfection (usually 28 days from date of disinfection), and the initials of the person performing the disinfection. Only competent, trained personnel should perform disinfection procedures.8
Maintaining a careful record of patients fit in each diagnostic lens is essential if a subsequent infection is diagnosed that was present during fitting. The information in the disinfection log can be used to contact patients who were later fit with that same diagnostic lens, to advise them of the potential risk of exposure.8
Label each disinfected diagnostic lens with the disinfection date. Repeat the disinfection process for soft and hybrid lenses every 28 days if the lens goes unused. Once a lens case or vial is opened for any reason (regardless of whether the lens is used), disinfect all items again.
While the guidelines minimize the risk of infection with multi-patient-use diagnostic lenses, the 2018 ISO Standard does not purport to inactivate prions and viruses. No standardized methods are currently available to do so in contact lenses.8 The ISO Standard recommends discarding lenses in cases of herpes simplex virus with corneal manifestations, hepatitis, human immunodeficiency virus, and adenovirus.
The Technical Report added two more conditions—patients who have prion disease and Acanthamoeba infection. In patients who have these conditions, it may be preferable to defer the fit until the condition has resolved or consider using empirical methods, noncontact methods (such as profilometry-guided designs) or impression-based methods of fitting.
THE CLASS SURVEY
The published 2018 ISO Standard and 2020 Technical Report have brought into question how well eye-care providers comply with cleaning and disinfection protocols and how academic institutions are educating optometry students. As a result, a group of contact lens educators from four institutions collaborated to create the Contact Lenses in Academia Solutions Study (CLASS) survey.11
One goal of the CLASS survey was to better understand the current cleaning and disinfection practices for multi-patient-use lenses at the schools and colleges of optometry. Additionally, awareness of—and adherence to—the procedures outlined in the 2018 ISO Standard for proper disinfection of multi-patient-use lenses was assessed. The investigators sent a request and survey link to educators identified as members of the Association of Optometric Contact Lens Educators. Snowball sampling was permitted in order to potentially include any faculty working in the contact lens department who may fit specialty lenses.
The sample, therefore, included contact lens, pediatric, myopia management, and dry eye faculty. A total of 91 educators initiated the survey. Responses were obtained from at least one respondent at each of the 25 North American schools and colleges listed as members of the Association of Schools and Colleges of Optometry.
Respondents’ most frequently reported role was “clinical faculty in a contact lens service” (21.5% of participants). Of the specialty contact lenses that were fit and handled regularly, the most common types were GP and scleral lenses (32.6% and 31.8%, respectively). Most respondents self-reported performing a “cleaning step” before disinfection for each lens category (97% of GP, 95% of scleral, 92% of hybrid, and 98% of specialty soft), but respondents were not necessarily following the 2018 ISO Standard recommendations.
Peroxide-based solutions were most frequently used to disinfect all materials (75% for GP, 80% for scleral, 83% for hybrid, and 82% for specialty soft). No participants reported using tap water to rinse soft or hybrid lenses; however, a small number reported using tap water to rinse GP and scleral lenses (4.1% and 2.6%, respectively). From this pool of contact lens educators, 14.5% reported being unaware of the 2018 ISO Standard for in-office disinfection of multi-patient-use diagnostic contact lenses.
An infographic was created to illustrate the proper disinfection steps and provide a visual aid for clinicians (Figure 5). The CLASS survey results indicate that contact lens educators are not necessarily performing the recommended ISO cleaning procedures before disinfection. This could be because the widely circulated infographic in Figure 5 specifies only the disinfection protocol and does not explicitly define the step involving digital rubbing with surfactant cleaner prior to the first disinfection soak in 3% ophthalmic hydrogen peroxide solution. Therefore, one way to improve proper compliance would be to include the initial cleaning step in the infographic (i.e., Step 1 above) and educate practitioners and students on best practices for disinfection.
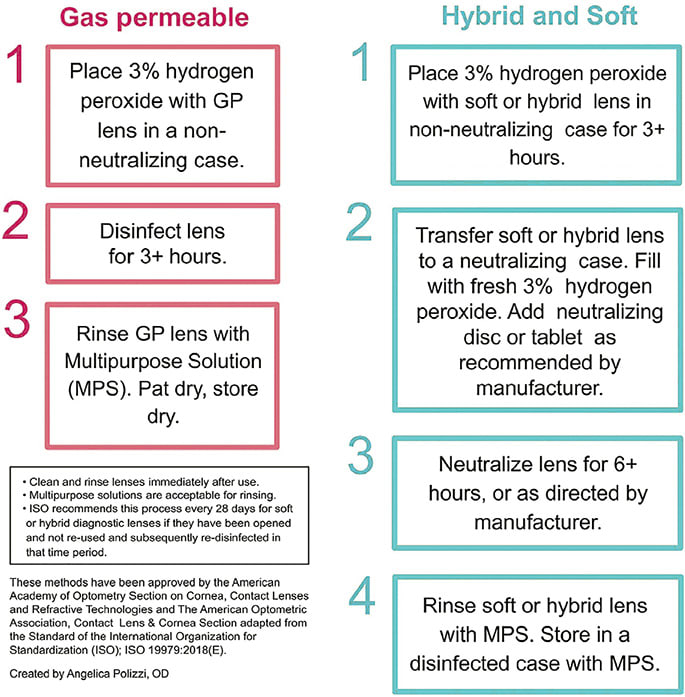
Although the CLASS survey indicates that tap water use is not very common in educational settings, the tendency to rinse GP and scleral lenses in tap water (for both eyecare practitioners and patients) may stem from the prior endorsement of the practice as seen in the solution package inserts or labels. In recent years, the instructions have been updated to advise against this (Figure 6).

Some solution manufacturers have gone beyond discouraging tap water use and included more detailed information about the risk of water contamination of contact lenses and cases (Figure 7). However, it is unclear whether patients will actually take the time to read the “fine print” on their contact lens solution instructions, so it is incumbent on practitioners to reeducate all patients, new and seasoned, on the best practices for lens care and handling.

For educators, this means it is vital that we instruct students and technicians on the best practices for lens care and advise on how to educate patients to phase out these unsafe practices. Reminders should be incorporated in each annual examination and follow-up appointment with attention to the risk of sight-threatening complications due to water exposure. Practitioners can suggest appropriate alternatives, such as rinsing with sterile saline or MPs while also emphasizing that homemade saline, bottled water, and distilled water are never recommended for rinsing any type of contact lens.6,12,13
APPROACH TO IMPLEMENTATION
Proper implementation of these processes in the clinic requires training of any practitioners and staff who will participate in the process. Ideally, there will be written protocols (e.g., checklists and flowcharts) and follow-through to ensure that proper procedures are followed. Any written instructions can be modeled after the ISO Standard along with the guidelines in Figure 5.
Practitioners should include the initial digital rub-to-clean step outlined in the 2018 ISO Standard that occurs after lens removal from the patient and before the initial soak in 3% ophthalmic hydrogen peroxide solution. Also, consider easing the burden of disinfection created by high-volume fitting (or such that we experience in a clinical laboratory setting) through the use of silicone ice cube trays with three slots across.
Silicone has excellent compatibility with hydrogen peroxide (10% concentration) for up to 48 hours, according to the Cole Parmer chemical compatibility database.14 According to Cole-Parmer, some acrylonitrile butadiene styrene plastics, low density polyethylene plastics, polycarbonate, and polypropylene also retain excellent compatibility with 10% hydrogen peroxide.14
The exact composition of the plastics used in diagnostic lens cases may vary by manufacturer, but the authors note anecdotal instances of plastic lens cases cracking after repeated use or soak times of > 48 hours (the limit suggested by Cole-Parmer for this interaction), even with the lower concentration of hydrogen peroxide found in contact lens solution (Figure 8).

A silicone tray has the potential to withstand wear over repeated rounds of disinfection. In our three-slot silicone trays, the far-left slot is used for hydrogen peroxide disinfection (three hours), the middle slot serves as a holding area for the original contact lens case while it is disinfected, and the right-hand slot either holds the rubber stopper (if used) or serves as a holding area for the neutralizing case (if needed) (Figure 9).

It is important to note that the neutralizing case is not required for disinfection of multi-patient-use GP lenses; the three-hour soak in hydrogen peroxide will suffice, followed by rinsing and drying. We also recommend marking one corner of the tray for orientation purposes, so that when two rows of lenses are together in a single neutralizing case, one can remember which lens is at the bottom of one row and which is at the top of the next row. We recommend using a consistent system (e.g., right on the top and left on the bottom and then the next right and then left). Also, be mindful that each neutralizing case with a catalytic disc should be discarded after use with 32oz (480mL) of solution; thus, patients using their lenses daily are advised to routinely replace their neutralizing cases after use of a single bottle of solution.15
To further improve compliance with—and knowledge of—the guidelines, involve trainees in the cleaning process. Post the guidelines for disinfection in contact lens clinic areas, ensure that gloves are readily accessible, and stock a large amount of ophthalmic hydrogen peroxide solution, neutralizing cases, sterile saline, and MPSs nearby. We also encourage the use of flat pack plastic cases in each examination lane to hold used lenses (e.g., after they contact the patient’s eye, but before they are digitally cleaned and transferred to the hydrogen peroxide solution to soak), so as not to contaminate the original diagnostic lens storage container. These disposable cases are discarded after use.
Specialty and custom contact lens manufacturers could enhance compliance with implementation of these updated procedures by printing the disinfection guidelines within each set’s fitting guide or by adding a QR code linking to the disinfection guidelines on the set’s outer case. Including repeated reminders to avoid contact with tap water would also be useful.
Updates to individual diagnostic lens storage cases would also be welcome, including designs that are both simple to disinfect and sturdy enough to withstand repeated cycles of contact with hydrogen peroxide. It would also be advantageous for any case labels to be waterproof so that lens parameters will not rub off or become obscured with repeated exposure to liquids (e.g., vinyl stickers). The more individual diagnostic lenses can be easily distinguished from one another (e.g., by the color of lens material or via laser etchings of lens parameters), the less likely lenses are to be mixed up during the disinfection process.
Finally, the burden of lens disinfection could be eased by supplying several units of the most commonly used lenses in each fitting set so that they can either be applied bilaterally and simultaneously to the same patient, or the second lens can be used on a subsequent fitting the same day. The full disinfection cycle for a corneal or scleral lens takes, at minimum, three hours to complete; and for a hybrid or specialty soft lens, the disinfection cycle takes at least nine hours to complete.
The authors acknowledge that they are not attuned to which changes may be feasible for contact lens manufacturers to implement and that any change will take time and money. The academic contact lens laboratory setting also has demands that differ from those of other practice modalities, and we are content to use our ice cube tray system to implement the 2018 ISO Standard for the foreseeable future. CLS
Acknowledgments: The authors gratefully acknowledge their collaborators in the CLASS Study group: Michelle Man, OD; Erin Rueff, OD, PhD; and Kelsy Steele OD, MS.
REFERENCES
- Yee A, Walsh K, Schulze M, Jones L. The impact of patient behaviour and care system compliance on reusable soft contact lens complications. Cont Lens Anterior Eye. 2021 Oct;44:101432.
- Cardona G, Alonso S, Yela S. Compliance versus Risk Awareness with Contact Lens Storage Case Hygiene and Replacement. Optom Vis Sci. 2022 May;99:449-454.
- Dart JKG, Radford CF, Minassian D, Verma S, Stapleton F. Risk factors for microbial keratitis with contemporary contact lenses: a case-control study. Ophthalmology. 2008 Oct;115:1647-1654.
- Stapleton F, Keay L, Edwards K, Holden B. The epidemiology of microbial keratitis with silicone hydrogel contact lenses. Eye Contact Lens. 2013 Jan;39:79-85.
- Arshad M, Carnt N, Tan J, Stapleton F. Effect of Water Exposure on Contact Lens Storage Case Contamination in Soft Lens Wearers. Cont Lens Anterior Eye. 2019 Sep 1;42:e34.
- Arshad M, Carnt N, Tan J, Ekkeshis I, Stapleton F. Water exposure and the risk of contact lens-related disease. Cornea. 2019 Jun;38:791-797.
- Li S, Bian J, Wang Y, Wang S, Wang X, Shi W. Clinical features and serial changes of Acanthamoeba keratitis: an in vivo confocal microscopy study. Eye (Lond). 2020 Feb; 34:327-334.
- International Organization for Standardization. ISO 19979:2018(en) Ophthalmic optics – contact lenses – hygienic management of multipatient use trial contact lenses. 2018. Available for purchase at iso.org/obp/ui/#iso:std:iso:19979:ed-1:v1:en . Accessed Feb. 16, 2020.
- Sindt C, Bennett E, Szczotka-Flynn L, Sclafani L, Barnett M; American Academy of Optometry (AAO) Section on Cornea, Contact Lenses & Refractive Technologies, and The American Optometric Association (AOA) Contact Lens and Cornea Section. Technical Report: Guidelines for Handling of Multipatient Contact Lenses in the Clinical Setting. Optom Vis Sci. 2020 Aug;97:544-548.
- Szczotka-Flynn L. Intrapatient and Interpatient Contact Lens Disinfection in the Age of COVID-19. Eye Contact Lens 2022 Feb 1;48:57.
- Man M, Steele K, Rueff E, Satjawatcharaphong P, Sicks L. Educator Awareness of an Adherence to ISO Standards for Multipatient Use Diagnostic Contact Lens Disinfection. Poster presented at Academy 2022, San Diego. 2022 Oct 28.
- U.S. Food and Drug Administration. Contact Lens Solutions and Products. 2018 Jan 16. Available at fda.gov/medical-devices/contact-lenses/contact-lens-solutions-and-products . Accessed Sep. 12, 2022.
- U.S. Food and Drug Administration. Focusing on Contact Lens Safety. 2019 Oct 16. Available at www.fda.gov/consumers/consumer-updates/focusing-contact-lens-safety . Accessed Sep. 29, 2022.
- Cole-Parmer. Chemical Compatibility Database. Available at coleparmer.com/chemical-resistance . Accessed July 28, 2022.
- Novartis. Clear Care Plus with HydraGlyde Package Insert, CCPlus_wHG_PI_InsideCarton. 2019. Available at clearcaresolution.myalcon.com/sites/g/files/rbvwei601/files/2019-05/CCPlus_wHG_PI_InsideCarton.pdf . Accessed July 28, 2022.



