The original treating physician diagnosed a corneal abrasion and began prophylactic topical antibiotic t.i.d. Several days later, a 2mm x 3mm painless central defect without subepithelial infiltrate or anterior chamber reaction (Figure 1) was observed and a fourth-generation topical fluoroquinolone was prescribed. We saw this patient on the fourth visit and noted a 2mm upper lid ptosis and a 1.5mm x 1mm central defect surrounded by grade 4+ SPK.
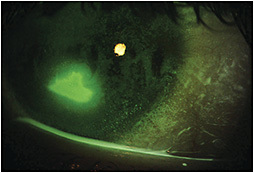
The patient was still without pain, which is uncharacteristic for such a lesion, so a cotton wisp test was performed that indicated no sensation in any of the five areas tested. Additional questioning revealed that the child had longstanding photophobia of unknown cause and had experienced a case of herpes simplex keratitis in that same eye as a young child. Her medical history was also significant for VATER syndrome, characterized by at least three developmental defects in the vertebrae, anus, trachea, esophagus, or renal system. Based on the poor-healing epithelial defect and absent corneal sensitivity, she was diagnosed with stage 3 neurotrophic keratitis (NK) and was started on an eight-week course of topical ophthalmic cenegermin solution.
NK is a degenerative condition caused by impairment of trigeminal nerve innervation and decreased corneal sensation. In early stages, breakdown of the epithelium occurs, which can progress to ulcer formation, corneal melting, and perforation (Semeraro et al, 2014). The biggest red flag is the absence of pain where pain should be otherwise anticipated. NK can develop alongside any condition associated with damage to the fifth cranial nerve, including herpetic keratitis, ophthalmic surgery or injury, chronic use of certain topical medications, diabetes, multiple sclerosis, and a variety of congenital syndromes (Sacchetti and Lambiase, 2014). At the time of this writing, no association has been made between VATER syndrome and NK.
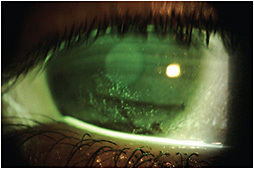
Full corneal defect resolution occurred five weeks into treatment and only scattered SPK remained after eight weeks (Figure 2). Amniotic membrane therapy was briefly considered, but given the age of the patient and need for vision correction, scleral lenses (SLs) were considered the best option. She began daily SL wear immediately after completing the full course of topical ophthalmic cenegermin and her SPK has continued to improve. The therapeutic benefits of SLs for patients with ocular surface disease are well documented (Pullum et al, 2005; Harthan and Shorter, 2018; Witsberger and Schornack, 2021).
Currently, there is a major lack of approved treatment options for NK, although several promising therapeutic alternatives are on the horizon (Dohlman et al, 2021). For now, NK management focuses on preventing disease progression and promoting corneal healing (Sacchetti and Lambiase, 2014). Clinical efficacy of cenegermin can persist for up to 48 months, although corneal defects may recur in as little as 12 to 36 months (Bruscolini et al, 2022). The patient will be followed closely. Thankfully, for now, she is doing well. CLS
REFERENCES
- Semeraro F, Forbice E, Romano V, et al. Neurotrophic keratitis. Ophthalmologica. 2014;231:191-197.
- Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol. 2014 Mar;8:571-579.
- Pullum KW, Whiting MA, Buckley RJ. Scleral contact lenses: the expanding role. Cornea. 2005 Apr;24:269-277.
- Harthan JS, Shorter E. Therapeutic uses of scleral contact lenses for ocular surface disease: patient selection and special considerations. Clin Optom (Auckl). 2018 Jul;10:65-74.
- Witsberger E, Schornack M. Scleral Lens Use in Neurotrophic Keratopathy: A Review of Current Concepts and Practice. Eye Contact Lens. 2021 Mar;47:144-148.
- Dohlman TH, Singh RB, Dana R. Advances in the Medical Management of Neurotrophic Keratitis. Semin Ophthalmol. 2021 May;36:335-340.
- Bruscolini A, Marenco M, Albanese GM, Lambiase A, Sacchetti M. Long-term clinical efficacy of topical treatment with recombinant human nerve growth factor in neurotrophic keratopathy: a novel cure for a rare degenerative corneal disease? Orphanet J Rare Dis. 2022 Feb;17:57.





