WHEN FITTING SCLERAL lenses, the understanding of ocular and scleral shapes is essential. Equally important, however, is understanding the ocular surface environment and tear film. Even if perfect scleral lens alignment is achieved, an unstable ocular surface can lead to significant challenges with lens fitting and successful wear.
Management of ocular surface disease (OSD) is complex. Even though scleral lenses have demonstrated the ability to treat and rehabilitate compromised ocular surfaces,1,2 they are not typically the first-line treatment. Therapies are usually individualized for patients, beginning with more basic and broadly available options and eventually leading to more specialized and invasive treatments if indicated.
A recent study on treatment for OSD3 surveyed 778 practitioners who have scleral lens prescribing experience. The results showed that lubricant eye drops were most commonly used, followed by meibomian gland expression, topical cyclosporine or lifitegrast, topical steroids, punctal plugs, and finally scleral lenses. This survey-based study found that 45% of participants ranked scleral lenses as their sixth, seventh, or eighth treatment based on median overall rank.3 This aligns with findings from the Tear Film & Ocular Surface Society’s Dry Eye Workshop II, which recommends scleral lenses in the third or fourth stage of dry eye disease treatment.4
Given the complexity of scleral lens care and handling by patients, as well as fitting challenges for eyecare practitioners, it makes sense that scleral lenses would be considered a mid-ranked treatment option for managing OSD. There are many obstacles to maintaining a smooth, debris-free, and well-fitting lens over a compromised ocular surface. So, to optimize scleral lens fittings for the treatment of OSD, one must first understand the etiology of the disease.
The reality is that systemic disease has a tremendous influence on the eye—its shape, structure, and tear film qualities. Systemic diseases affect the entire body, and the eye, encased in mucus membranes, with a highly innervated cornea and the need for a healthy and robust tear film, is often one of the structures most impacted by systemic imbalance.
SJÖGREN’S
Sjögren’s is an autoimmune disorder affecting the exocrine glands. The population prevalence has been estimated to be 0.5% and is more common in females than males.5 The disorder can exist independently or be associated with other autoimmune conditions such as rheumatoid arthritis, systemic lupus erythematosus, or progressive systemic sclerosis. Involvement of the salivary and lacrimal glands contributes to dry mouth and dry eyes, respectively. The presence of ocular and oral symptoms, along with objective signs of dry eye and salivary gland involvement, abnormal laboratory Anti-SS-A or Anti-SS-B, ANA, and IgM rheumatoid factor tests, aid in diagnosis of the condition.5,6 Topical therapy works to improve moisture and decrease inflammation, while systemic therapy includes steroidal and non-steroidal anti-inflammatory agents, disease-modifying agents, and cytotoxic agents to address manifestations in the skin, lungs, heart, kidneys, and nervous system.
Dry eye in Sjögren’s manifests as inflammation of the lacrimal gland and ocular surface (Figure 1). Ocular surface challenges include insufficient tear production from the lacrimal gland, thinned and unstable tear film, and the presence of mucus strands and filaments.6 It is important to utilize vital dyes, such as sodium fluorescein, rose bengal, and/or lissamine green during evaluation to fully appreciate changes in the ocular surface that may be overlooked with only white light. A Wratten filter used with cobalt blue light will also enhance visualization of the ocular surface after sodium fluorescein instillation.
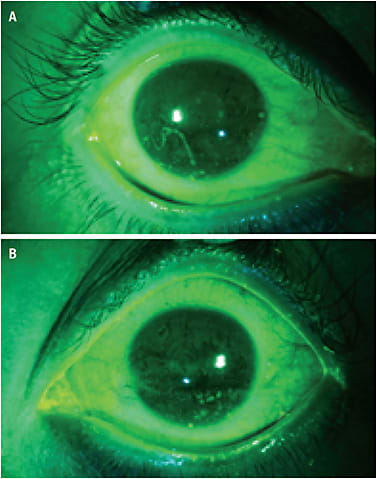
Therapies aimed at minimizing these effects include tear substitutes, topical corticosteroids and cyclosporine, dietary omega-3 fatty acids, oral secretagogue therapy, punctal plugs, autologous serum tears, topical N-acetylcysteine, soft bandage contact lenses, and scleral lenses. Various therapies may be used concurrently to maximize treatment effectiveness.
Lens design and fitting in patients who have Sjögren’s is usually straightforward because ocular shape and eyelid anatomy are generally not irregular. Larger diameter lenses can be successful with these patients, as they cover more of the exposed ocular surface. However, the compromised surface makes lens wear and daily management quite difficult. It may be common for patients to experience visual fluctuations due to poor lens wetting and blur caused by mucus films developing on the front surface of the lens.
Blinking may help to improve vision temporarily by moving debris away, but it may be necessary to either wipe the surface with a sterile saline-moistened cotton swab or remove the lens entirely to clean it. In addition to cleaning the lens with approved products, patients should also rinse and clear the ocular surface of mucus and debris before lens reapplication.
Some scleral lens wearers may have to wipe or clean their lenses multiple times a day. While using plastic materials with lower wetting angles may enhance lens wetting, one may also consider placing a polyethylene glycol (PEG)-based polymer coating on the lens to improve comfort and wettability.7
GRAFT-VERSUS-HOST DISEASE
Graft-versus-host disease (GVHD) is a complication of allogeneic hematopoietic stem cell transplantation (allo-HSCT) in which donor T cells view recipient antigens as an unknown threat and subsequently attack. This complication is the leading cause of late morbidity and mortality after allo-HSCT, but for patients who have hematologic malignancies, metabolic and immunodeficiencies, or bone marrow failure syndromes, an allo-HSCT may be the only cure.8,9
Acute GVHD onset occurs within the first 100 days after allo-HSCT, while chronic GVHD involves characteristics that appear at least 100 days after transplant. In modern times, GVHD classification is based on clinical presentation rather than time of symptom onset.9 GVHD acutely leads to cutaneous, gastrointestinal, and hepatic involvement, while chronic GVHD mainly affects the skin, mucous membranes, eyes, liver, lungs, soft tissues, and musculoskeletal system; the chronic disease has greater morbidity rates and more often leads to quality-of-life issues.9
The frequency of chronic GVHD ranges from 30% to 70%,9 while the frequency of ocular GVHD (oGVHD) has been reported at 40% to 60% following allo-HSCT. oGVHD can manifest with meibomian gland dysfunction (MGD) and meibomian gland atrophy.10 The development of corneal and conjunctival inflammation may contribute to epithelial erosions, filamentary keratitis, and even corneal stromal ulceration, melts, and perforation. Lid fibrosis and atrophy with keratinization may lead to entropion or ectropion, and superior limbic keratoconjunctivitis has even been reported as a manifestation of oGVHD.11,12
These ocular complications may lead to debilitating pain, decreased vision, photophobia, and significantly decreased patient quality of life.11 During systemic GVHD flare-ups, oral immunosuppressants may be utilized to control inflammation in the body and eyes, and may also enhance scleral lens wear.13
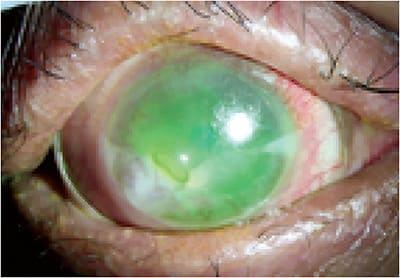
While artificial tears, topical steroids and cyclosporine, autologous serum tears, punctal plugs, and protective goggles may help improve symptoms in mild-to-moderate cases of oGVHD, scleral lenses have been shown to drastically improve ocular comfort and vision in more advanced cases.10-12,14 However, due to the severely compromised mucus membranes around the eye and disrupted tear film related to MGD, lenses may become soiled and mucus-coated (Figure 2) on the eye, after even brief periods of wear. Similar to maintenance protocols recommended for Sjögren’s lens wearers, patients who have oGVHD may also need to routinely swab and/or remove their scleral lenses for cleaning multiple times a day.

Interestingly, some patients feel such significant dry eye relief from lens wear that they don’t mind cleaning their lenses throughout the day to optimize vision as needed. Some might actually tolerate blurred vision rather than suffer from ocular pain. One study even reported that 56% of those wearing scleral lenses wished that the lenses had been recommended sooner, and that the most common reason patients cited for not wearing the lenses was that they had never heard of them.14
STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSIS
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are life-threatening diseases characterized by skin detachment and blisters, with high mortality rates and both short-term and long-term morbidities.15 SJS/TEN are predominantly induced by medications such as antibiotics, allopurinol, nonsteroidal anti-inflammatory drugs, and antiepileptic drugs; infection with Mycoplasma pneumoniae or herpes simplex virus has also been recognized in its pathogenesis. The incidence rate of this delayed-type drug hypersensitivity reaction in the U.S. has been reported at 1.58 to 2.26 cases/million people.15
SJS and TEN represent different stages of the same mucocutaneous reaction and are classified by the percentage of skin detachment area, with SJS having < 10% skin detachment area, SJS/TEN overlap with 10% to 30%, and TEN with > 30% involvement.15 Malaise and fever often precede the onset of skin manifestations by a few days, and there is a typical latency of 4 to 28 days following initiation of the implicated drug.16
Most patients who have SJS/TEN develop mucosal involvement of the mouth, genitalia, and eyes. Ocular involvement is common, and management may include artificial tears, removal of pseudomembranes, lysis of symblepharon, debridement of loosened epithelium, topical antibiotics to prevent secondary infection, topical corticosteroids to prevent scar formation, and cycloplegic drops to relieve pain, photophobia, and ciliary spasm. Amniotic membrane transplantation has also been shown to be effective in the acute and chronic stages of SJS/TEN.16
Upon clinical presentation in the chronic stages, eyecare practitioners may appreciate compromised tear film, mucus strands, filamentary keratitis, corneal staining, neovascularization, scarring, limbal stem cell deficiency, bulbar and palpebral conjunctival scarring, symblepharon (Figure 3), conjunctivalization of the cornea (Figure 4), and irregular lid margins with trichiasis and distichiasis (Figure 5).



In advanced stages, epithelial breakdown may lead to persistent corneal epithelial defects (Figure 6), as well as keratinization of the conjunctiva and cornea (Figure 7). Keratinization involves the development of a waxy and hardened tissue layer that ultimately acts to protect and shield the eye. Unfortunately, although this process may subdue acute eye pain and sensitivity, it also leads to corneal opacification and severe loss of vision.

With total corneal opacification, the only option for possible visual improvement would be an artificial cornea device, with the Boston keratoprosthesis. Ultimately, eyes in SJS/TENS are very sensitive and reactive to environmental changes. Inflammation and scarring are easily triggered by abrasions, infections, and surgical interventions, including cataract surgery.
SJS/TEN severely alters the mucus membranes of the ocular surface and changes the shape and structure of the cornea, conjunctiva, and eyelids. Due to these anatomical changes, scleral lens fitting can be extremely problematic. Despite these fitting challenges, however, scleral lenses have proven to be a viable option for improving vision and ocular function in eyes unresponsive to traditional therapies.17,18
In eyes with symblepharon, it may be necessary to use a smaller-diameter lens to minimize impingement on the stringy bands of scar tissue. Regardless of symblepharon formation, toric, quadrant-specific, image-guided, and impression-molded scleral lens designs may be helpful to best align the cornea in primary gaze. Any movement of the eye will change the surface contour, especially in the presence of conjunctival scarring and symblepharon. Gaps develop transiently between the rigid plastic lens and bulbar conjunctival tissue, which allow for unwanted entry of proteins, mucus, and oily particles into the fluid reservoir.
Therefore, SJS/TEN patients need to be prepared for debris movement and midday fogging throughout the day. The use of thicker ophthalmic gels in place of traditional saline to fill the lens chamber may help to reduce debris accumulation under the lens, but frequent saline rinsing and re-cleaning of the lens and ocular surface are often unavoidable.
Tear film abnormalities also contribute to problems with surface wetting and deposits. Still, many patients persist in wearing their lenses due to the immense relief they bring.
FITTING TIPS & TRICKS
FOR LENS EVALUATION AND FITTING
- Do not use anesthetic during initial evaluation. Patients must feel the effect of the lens in the eye to provide feedback on whether or not comfort is improved.
- Allow the diagnostic scleral lens to settle for 15 minutes in office, at minimum. Patients must be able to adapt to having lenses on their eyes. The edge landing profile will change over time as it settles into the conjunctiva.
- Initial settling will provide insight into potential tear film, surface wetting, and debris challenges.
- If effects of the lens trial are inconclusive during the first consult, schedule the patient for re-evaluation on a different day. Dry eye fluctuates often, and one visit may not be representative of the patient’s overall situation.
- Allow for sufficient time to explain the role of a scleral lens and the maintenance and care required.
FOR LENS TRAINING
- Explain or demonstrate application and removal process to the patient with videos or models.
- Practice lid spread with dry fingers and dry eyelids in front of a mirror.
- Offer tools to assist with lens application. Various plunger stands and lights for fixation are available.
- Lubricate the ocular surface sufficiently before attempting the lid spread, as it is difficult for patients to open their eyes wide and for long periods due to dryness.
- Dim the lights if the patient has photophobia.
- Take breaks.
- Encourage the patient to bring a friend or family member, so they might be able to help at home, if needed.
- A training session should not last more than 60 minutes, as fatigue and frustration will set in. Reschedule for additional training sessions as needed. Ask the patient to practice lid spread and head positioning at home to better prepare for the next visit.
FOR DAILY LENS WEAR
- Prioritize lid hygiene in the morning before lens application and after lens removal at night.
- Use non-preserved saline to rinse the eyes midday to flush away debris and keep the lenses clean.
- Use non-preserved artificial tears over the lenses for comfort as needed during wear.
- Consider utilizing a polyethylene glycol-based polymer coating on the lenses to improve surface wetting; this coating tends to work better for mild to moderate dry eyes. Be aware of cleaning products contraindicated with this coating.
- Consider recommending a bi-weekly or monthly enzyme protein cleaner to better maintain the lenses.
- Ensure that patients are using the appropriate lens cleaning, filling, and storage solutions.
- Remind patients to never sleep in their lenses.
- Emphasize that routine follow-up care is necessary, even if their eyes feel fine.
CORNEAL DISORDER ASSOCIATED WITH OCULAR SURFACE AND SYSTEMIC DISORDERS
Keratoconus is not a systemic disease, but is often associated with conditions such as Down syndrome, Leber’s congenital amaurosis, Ehlers-Danlos syndrome, Noonan syndrome, and mitral valve prolapse.19,20 Additionally, this corneal thinning disorder has a strong association with atopic conditions, including asthma, eczema, and ocular allergies.19,20 Allergic conjunctivitis and elevated levels of serum immunoglobulin E17 likely contribute to the eye-rubbing compulsion in these patients.
A recent study of keratoconus patients21 demonstrated ocular surface alterations, including decreased tear breakup time (TBUT) values and greater goblet cell loss with more advanced disease. Given these associated ocular surface changes and challenges, surface wetting issues, mucus film and plaque deposition (Figure 8), and midday fogging are possible with scleral lens wear in keratoconus patients. Treatment of dry eye and ocular allergies may help to enhance overall lens condition and comfort.

CONDITIONS THAT AFFECT JOINTS AND FINE MOTOR CONTROL
Many disorders, such as rheumatoid arthritis, osteoarthritis, psoriatic arthritis, gout, and systemic lupus erythematosus have joint involvement.22 These conditions may contribute to deformation in the extremities and limit mobility of the shoulders, arms, hands, and fingers (Figure 9). These anatomical changes may negatively impact precise movements required for scleral lens application and removal.

Other neurodegenerative conditions, such as Parkinson’s disease and cerebellar disease, may lead to tremors and loss of fine motor control.23,24 Weakened muscles and loss of fine motor coordination may also lead to difficulty with lens maintenance and handling. While plunger stands and lighted devices can aid in the application of the scleral lens, often an assistant (family member, friend, or caretaker) may need to help the patient apply and remove their lenses.
CONCLUDING CLINICAL PEARLS
Eyecare practitioners need to understand the patient, and the patient’s underlying systemic disease, before treating their eyes. The provider and patient need to be aware that both eyes and the ocular surface may evolve as the patient’s disease changes, and that dry eye fluctuations will occur over time and with variations in medications.
This will ultimately affect scleral lens fittings, designs, routine wear, and the daily care and maintenance involved. It is also important to comanage with other medical specialists to coordinate care, and possibly consider medication adjustments when needed to stabilize the ocular surface.
In addition to understanding systemic disease and how it affects the ocular surface, scleral lens practitioners must also be ready for action in the clinic to best serve their patients. The “Fitting Tips & Tricks” sidebar on page 26 provides some practical tips for initial scleral lens evaluations and fittings, lens trainings, and daily lens wear for OSD patients. CLS
References
- Schornack MM, Pyle J, Patel SV. Scleral lenses in the management of ocular surface disease. Ophthalmology. 2014 Jul;121:1398-1405.
- Harthan JS, Shorter E. Therapeutic uses of scleral contact lenses for ocular surface disease: patient selection and special considerations. Clin Optom (Auckl). 2018 Jul 11;10:65-74.
- Shorter E, Fogt J, Nau C, Harthan J, Nau A, Schornack M. Prescription Habits of Scleral Lenses for the Management of Corneal Irregularity and Ocular Surface Disease Among Scleral Lens Practitioners. Eye Contact Lens. 2023 Feb 1;49(2):46-50.
- Jones L, Downie LE, Korb D, et al. TFOS DEWS II Management and Therapy Report. Ocul Surf. 2017 Jul;15(3):575-628.
- Fox RI. Sjögren’s syndrome. Lancet. 2005 Jul 23-29;366:321-331.
- Foulks GN, Forstot SL, Donshik PC, et al. Clinical guidelines for management of dry eye associated with Sjögren disease. Ocul Surf. 2015 Apr;13:118-132.
- Mickles CV, Harthan JS, Barnett M. Assessment of a Novel Lens Surface Treatment for Scleral Lens Wearers with Dry Eye. Eye Contact Lens. 2021 May 1;47:308-313.
- Hamilton BK. Updates in chronic graft-versus-host disease. Hematology Am Soc Hematol Educ Program. 2021 Dec 10;2021:648-654.
- Moreno DF, Cid J. Graft-versus-host disease. Med Clin (Barc). 2019 Jan 4;152:22-28.
- Jacobs DS, Carrasquillo KG, Cottrell PD, et al. CLEAR - Medical use of contact lenses. Cont Lens Anterior Eye. 2021 Apr;44(2):289-329.
- Magro L, Gauthier J, Richet M, et al. Scleral lenses for severe chronic GvHD-related keratoconjunctivitis sicca: a retrospective study by the SFGM-TC. Bone Marrow Transplant. 2017 Jun;52:878-882.
- Theophanous C, Irvine JA, Parker P, Chiu GB. Use of Prosthetic Replacement of the Ocular Surface Ecosystem Scleral Lenses in Patients with Ocular Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2015 Dec;21:2180-2184.
- Chiu GB, Theophanous C, Irvine JA. PROSE Treatment in Atypical Ocular Graft-Versus-Host Disease. Optom Vis Sci. 2016 Nov;93:1444-1448.
- Bligdon SM, Colarusso BA, Ganjei AY, Kwok A, Luo ZK, Brocks D. Scleral Lens and Prosthetic Replacement of the Ocular Surface Ecosystem Utilization in Ocular Graft-versus-Host Disease: A Survey Study. Clin Ophthalmol. 2021 Dec 25;15:4829-4838.
- Hasegawa A, Abe R. Recent advances in managing and understanding Stevens-Johnson syndrome and toxic epidermal necrolysis. F1000Res. 2020 Jun 16;9:F1000 Faculty Rev-612.
- Dodiuk-Gad RP, Chung WH, Valeyrie-Allanore L, Shear NH. Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: An Update. Am J Clin Dermatol. 2015 Dec;16(6):475-493.
- Heur M, Bach D, Theophanous C, Chiu GB. Prosthetic replacement of the ocular surface ecosystem scleral lens therapy for patients with ocular symptoms of chronic Stevens-Johnson syndrome. Am J Ophthalmol. 2014 Jul;158(1):49-54.
- Tougeron-Brousseau B, Delcampe A, Gueudry J, et al. Vision-related function after scleral lens fitting in ocular complications of Stevens-Johnson syndrome and toxic epidermal necrolysis. Am J Ophthalmol. 2009 Dec;148:852-9.e2.
- Santodomingo-Rubido J, Carracedo G, Suzaki A, Villa-Collar C, Vincent SJ, Wolffsohn JS. Keratoconus: An updated review. Cont Lens Anterior Eye. 2022 Jun;45:101559.
- Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998 Jan-Feb;42:297-319.
- Mirza E, Oltulu R, Oltulu P, Mirza GD, Okka M. Dry eye disease and ocular surface characteristics in patients with keratoconus. Saudi J Ophthalmol. 2022 Jul 11;36:117-121.
- Jacobson JA, Girish G, Jiang Y, Sabb BJ. Radiographic evaluation of arthritis: degenerative joint disease and variations. Radiology. 2008 Sep;248:737-747.
- Shahien M, Elaraby A, Gamal M, et al. Physical therapy interventions for the management of hand tremors in patients with Parkinson’s disease: a systematic review. Neurol Sci. 2023 Feb;44:461-470.
- Frei K, Truong DD. Medications used to treat tremors. J Neurol Sci. 2022 Apr 15;435:120194.




