THE COMPLEXITY OF DRY EYE and ocular surface disease can be overwhelming even to experienced practitioners. The variability in symptoms, extensive case history considerations, and numerous possible clinical signs all make diagnosis difficult. Treatment decision-making isn’t any easier; the wide array of available treatment options must be narrowed down to target each individual’s needs, and we often feel inadequate when patient symptoms persist.
These challenges, combined with increasingly busy optometric practices and a growing demand for dry eye care,1create the need for an efficient and effective dry eye protocol. The second Tear Film & Ocular Surface Society Dry Eye Workshop (TFOS DEWS II) report includes a comprehensive list of treatment recommendations, organized in order of disease severity.2
However, because of the multifactorial nature of dry eye disease, it can be useful to preemptively identify and reduce external causative factors and only then begin other prescribed treatments. After this, organizing published recommendations into subcategories that target specific aspects of the condition can help clinicians recall all facets of the disease that need to be addressed. Fortunately, recent advances in understanding, diagnosis, and treatment of dry eye disease are helping practitioners find solutions for these patients.
PATIENT-CONTROLLED FACTORS
Some contributing factors, such as older age, female sex, Asian ethnicity, and geographical location, cannot be modified.3 Modifiable factors are arguably more important to determine, as any successful attempt to reduce causes of dry eye that are driven by elective patient habits may help avoid further treatments. This can save time, money, and unnecessary medications and their potential side effects.
Evaluation of the patient’s home and work environments can reveal potential etiologies for dry eye symptoms. Temperature; humidity; windy, polluted, perfumed, or noxious environments; and exposure to particulate matter should be investigated. Higher humidity and warmer temperatures tend to reduce dry eye symptoms, except in cases in which these conditions lead to increased microbial growth or particulate matter.4,5
Installing home humidifiers, particularly in the bedroom, can be helpful for most dry eye patients, so long as the humidity isn’t set too high and the humidifier/filters are cleaned regularly. In addition, smoking should be strongly discouraged.
Proper eye protection in harmful environments is important, as well as the avoidance of direct air flow onto the eyes. A common example of this is an overhead ceiling fan that circulates air while the patient is asleep; if it cannot be turned off, a sleep mask or moisture chamber goggles should be worn at night.
Continuous positive airway pressure (CPAP) machines are also notorious for leaking air into a patient’s eyes at night. If a proper fit that avoids this cannot be obtained, then nighttime eye masks or protective ointments should be considered.
A patient’s diet can have a considerable influence on systemic inflammation and, therefore, dry eye disease.1 Poor dietary choices, increased preservatives, artificial ingredients, and insufficient intake of essential nutrients are all implicated in undesirable gut biomes and obesity, both of which correlate well with systemic inflammation.1
Vitamin deficiencies are implicated in some dry eye studies, particularly vitamins A, B12, C, and D.6 Patient efforts to improve their diet and exercise or add supplementation with omega-3 fatty acids and multivitamins may be of benefit, and clinicians should test for deficiencies when suspected.7
Digital device use can be significantly detrimental to ocular surface health.8 It has been hypothesized that the mechanisms behind this include decreased blink rates, incomplete blinks, and possibly corneal phototoxicity.8 With increased device usage in nearly every age group, this should always be discussed as a modifiable risk factor for dry eye.
In young patients without other identifiable causative factors, excessive screen time is a primary cause of symptoms. When screen time is unavoidable, changes may be implemented to reduce its effects on the eyes. Lowering screens into down gaze, wearing spectacles to block ambient air flow, using non-preserved artificial tears while working, and taking frequent breaks are all beneficial habits that can mitigate dry eye-related symptoms.
Makeup use has notable associations with dry eye disease. Injury to the ocular surface from makeup application and removal, disruption of the tear film by particulate matter, microbial exposure to contaminated products, and toxicity from numerous chemical ingredients and preservatives are all likely contributors to the chronic inflammatory cycles of dry eye disease.9
When patients are firmly committed to makeup wear, it is important to encourage them to use eye-friendly products, avoid using them near the meibomian gland orifices (on the “waterline” posterior to the eyelashes), remove their makeup before bed, avoid eyelash extensions, and discard and replace used makeup at appropriate intervals.
Contact lens wear is another often non-negotiable but detrimental habit of the dry eye patient. Theorized to upset tear film homeostasis,10 contact lens wear is also being studied closely for evidence that it may also cause and/or worsen dysfunction of the meibomian glands, possibly by inducing changes in their structure.11
Many patients recognize that their dry eye is exacerbated by contact lens wear, but refuse to wear only spectacles. The effects of soft contact lens wear on the ocular surface can be reduced by educating patients regarding compliance with proper wear and care, discontinuation of any overnight wear, limiting contact lens wear overall, and refitting to daily disposable lenses when possible.12
In some patients, GP lenses may offer benefits that soft lenses do not. Overnight orthokeratology may be an option for many patients struggling with dryness in soft contact lenses. Studies have found that this modality improved symptoms of dryness, injection, goblet cell density, and conjunctival staining compared to soft contact lenses.13,14
Furthermore, scleral contact lenses may be utilized to not only reduce dry eye symptoms but also to protect and help heal the ocular surface.15 Scleral shells/prosthetics are an excellent option for patients who have moderate to advanced dry eye disease when the fitting challenges and costs involved are worth the intervention.
However, the possible detrimental effects of contact lens wear on meibomian gland health may increase with rigid lens wear, although much more research is needed in this area.16,17
When clinical time is limited, a brief handout may suffice to alert patients to positive changes in these external factors that they themselves can make. An example of this can be found in Table 1.
Once modifiable factors are addressed, the clinician can move to examination and treatment decision-making. Having patients complete a symptoms survey as well as performing objective testing is useful to help identify problem areas that need to be addressed. Additionally, you may be required to provide evidence of the need for prescription drug therapies to insurance companies on prior authorization forms.
The most fundamental objective evidence of the presence of dry eye disease may be tear film hyperosmolarity2 and corneal and conjunctival staining (using fluorescein or vital dye staining, as in Figure 1), although neither signals for specific treatment options by itself. To simplify the many other clinical signs to look for and the corresponding treatments that address the specific issues found, the acronym MOIST can be used: Moisturize, Open/unblock, Inflammation control, Systemic concerns, and Tidying up the lids and lashes.

MOISTURIZE
Assessment and treatment of insufficient tear quantity is often the first pillar of dry eye therapy. Testing for this may include noting tear meniscus height and performing Schirmer’s or phenol red thread testing.
Recommending name brand, non-preserved artificial tears, gels or ointments, a tear stabilizer such as perfluorohexyloctane, and/or punctal plugs provides a boost to tear volume and moisture to the ocular surface. In more severe cases, oral secretagogues, autologous serum drops, or scleral lenses may be offered.
OPEN/UNBLOCK
Improving tear quality by addressing lipid deficiency/evaporation is crucial in the management of the majority of dry eye patients. Meibography (Figure 2), investigative manual expression, interferometry, and tear breakup time (TBUT) can help identify the extent of this problem in each patient. In particular, meibography can be a powerful teaching tool to help convince patients of the need to address this aspect of their symptoms.

Treatment options include at-home warm compresses, in-office gland warming and expression procedures, oral tetracycline, and oral omega-3 fatty acid supplementation. Research regarding light therapies like intense pulsed light (IPL) and/or low-level light therapy (LLLT) is promising;18 indeed, for many offices this is becoming part of the standard treatment protocol for appropriate candidates.
INFLAMMATION CONTROL
One of the most important parts of long-term dry eye control is limiting the effects of inflammation.2 Early intervention can prolong ocular surface health in the presence of this chronic complication of dry eye disease.
Beyond looking for injection, chemosis, or other signs of inflammation on examination, in-office testing for matrix metalloproteinase-9 enzyme (MMP-9) can be useful to detect early inflammatory disease.19 Addressing inflammation may include use of topical steroids, cyclosporine A, and lifitegrast20,21 as well as oral therapies that target underlying systemic inflammation when present.
SYSTEMIC CONSIDERATIONS
In addition to ocular and environmental etiologies for dry eye, there are numerous systemic issues to consider. Allergies are a common comorbidity with dry eye, and a possible contributing factor. Unfortunately, systemic treatment for allergies in the form of oral antihistamines can exacerbate ocular dryness.22 Efforts to control allergen exposure and reduce the need for oral antihistamines may be helpful.
Other systemic medications have been linked to dry eye symptoms, including anticholinergics, antidepressants, antipsychotics, isotretinoin, chemotherapeutics, antithyroids, antihypertensives, corticosteroids, diuretics, hormonal therapy, and more.22 These medications may not have acceptable alternatives and generally should not be discontinued, especially without consulting the patient’s primary care physician.
Topical medications may cause dry eye symptoms as well, with ocular hypotensives used in glaucoma management being commonly implicated.23 When this is an issue, laser or surgical treatment for glaucoma may need to be considered.
Numerous systemic conditions are associated with dry eyes, particularly connective tissue diseases such as rheumatoid arthritis, autoimmune diseases (e.g., Sjögren’s syndrome or lupus), rosacea, peripheral artery disease, and thyroid disease.24 Aggressive systemic treatment of these conditions is critical for controlling the ocular signs and symptoms of dry eye, as topical therapies alone are often insufficient.
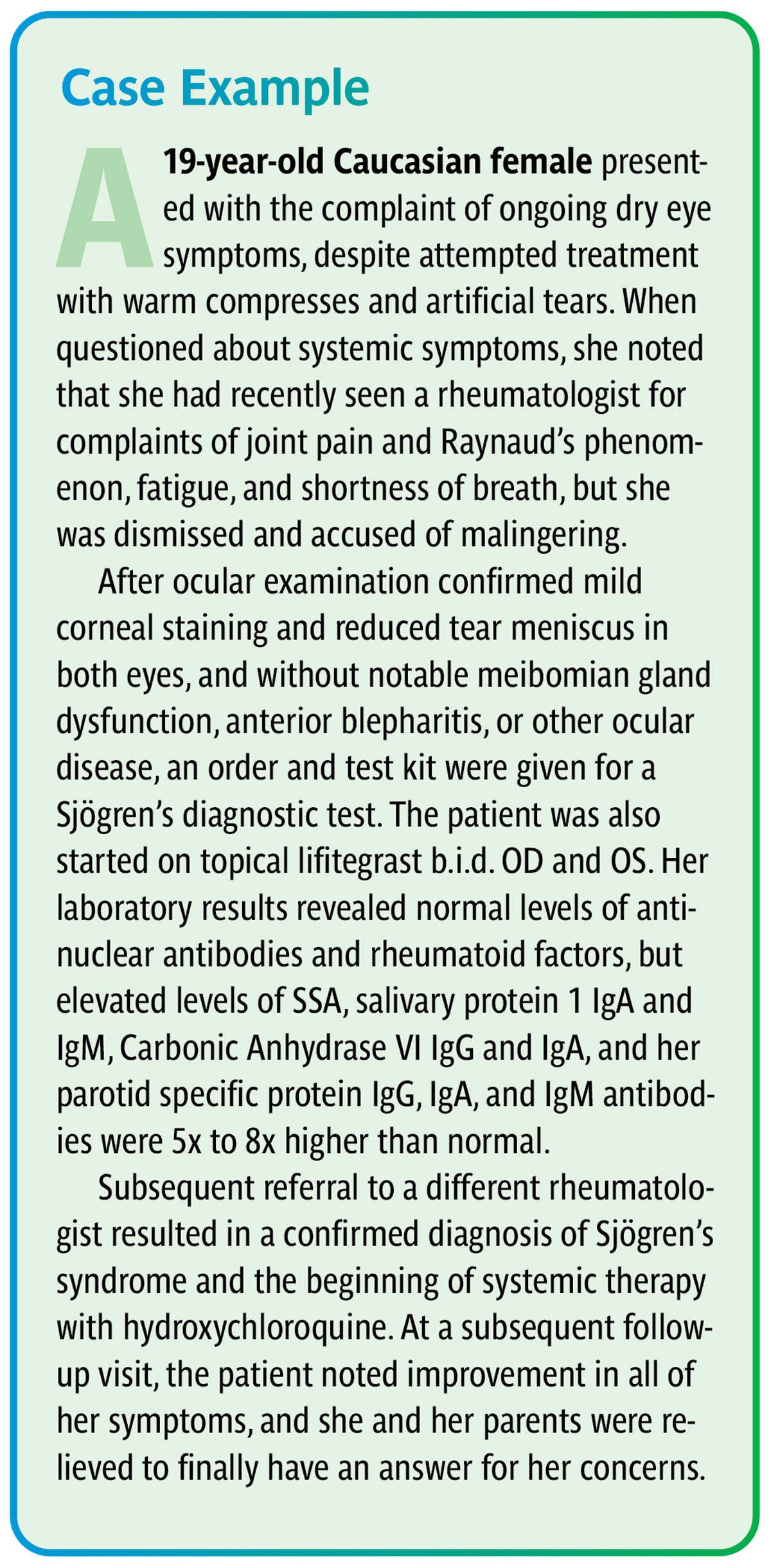
A consult with the patient’s primary care physician and referral for an endocrinology, rheumatology, and/or autoimmune workup should be performed before significant disease progression occurs. Unfortunately, scheduling patients with some specialists can be difficult to achieve in a timely manner; when this is the case, a panel of laboratory tests can be ordered by the eyecare practitioner to determine whether referral is necessary. An example of this is shown in the case at left (see sidebar below).
This type of workup may include testing for anti-Ro/SSA and La/SSB biomarkers, antinuclear antibodies (ANA), rheumatoid factors, and salivary gland, carbonic anhydrase VI, and parotid secretory protein antibodies.25 Providing confirmation of specific systemic concerns and justifying the need for further workup can expedite scheduling a patient’s appointment with the appropriate specialist.
TIDYING UP THE LIDS AND LASHES
A clean and healthy eyelid is essential for tear film homeostasis. Identifying structural problems like floppy eyelid syndrome, lagophthalmos, and ectropion, as well as lid wiper epitheliopathy and telangiectatic signs of ocular rosacea, can lead to more accurate symptom management.
Blepharitis should be aggressively treated with a recommended lid hygiene product (e.g., over-the-counter cleansers, hypochlorous acid sprays, tea tree oil preparations, etc.), topical or oral antibiotics, and Demodex therapies such as topical lotilaner as needed.26 In-office blepharoexfoliation may provide relief by removing microbial biofilm from the lid margins in many cases (Figure 3).27

Two other conditions that the dry eye practitioner should also consider concern abnormal neuronal function in the cornea: neurotrophic keratitis and neuropathic pain. Neurotrophic keratitis can result from numerous etiologies that affect the trigeminal nerve anywhere along its pathway; however many cases result from direct corneal insult (e.g., from herpes simplex keratitis, severe blepharitis, dry eye, or rosacea).28
Patients who have this condition have reduced corneal sensitivity, often accompanied by extreme corneal signs ranging from persistent epithelial loss and stromal haze to ulceration in its final stage. Treatment in early stages include standard dry eye therapies to protect the cornea; however, more advanced therapies, such as topical nerve growth factors or nerve grafting/neurotization, may be required if early intervention is not sufficient.
Neuropathic pain presents in nearly the opposite manner: patients complain of significant dry eye and pain (increased corneal sensitivity), but have mild, if any, clinical signs of dry eye (“all pain, no stain”).29 Additionally, their symptoms persist despite standard dry eye therapy.
While poorly understood, it is suspected that the etiology for this seemingly unprovoked pain is driven by neurosensory abnormalities and changes in synaptic pain signaling along either the central or peripheral nervous system, or both.30Treating these patients is challenging, requiring both topical corneal rehabilitation and systemic pain management.
Unfortunately, when dry eye progresses from a mild nuisance to a chronic inflammatory disease, treatment will need to be ongoing and consistent, and patient compliance is often a struggle. Regular discussion is important to reiterate the need for aggressive and early treatment to prevent significant progression of this disease and the reduced efficacy of simpler and inexpensive therapies.
It is important to monitor dry eye patients through additional follow-up visits, even in mild and moderate cases, and education should be given repeatedly regarding the importance of adjusting the patient’s environments, habits, and commitments to fight their condition. Despite these challenges, a practitioner utilizing evidence-based practice guidelines to diagnose and employ both old and new treatment options can provide relief to even the most advanced dry eye patients.
REFERENCES
1. Tavakoli A, Flanagan JL. Dry eye disease: an (in)convenient truth. Clin Exp Optom. 2022 Mar;105:222-229.
2. Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II Report Executive Summary. Ocul Surf. 2017 Oct;15:802-812.
3. Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017 Jul;15:334-365.
4. Alves M, Asbell P, Dogru M, et al. TFOS Lifestyle Report: Impact of environmental conditions on the ocular surface. Ocul Surf. 2023 Jul;29:1-52.
5. Huang A, Janecki J, Galor A, et al. Association of the Indoor Environment With Dry Eye Metrics. JAMA Ophthalmol. 2020 Aug 1;138:867-874.
6. Fogagnolo P, De Cilla’ S, Alkabes M, Sabella P, Rossetti L. A Review of Topical and Systemic Vitamin Supplementation in Ocular Surface Diseases. Nutrients. 2021 Jun 10;13:1998.
7. Pellegrini M, Senni C, Bernabei F, et al. The Role of Nutrition and Nutritional Supplements in Ocular Surface Diseases. Nutrients. 2020 Mar 30;12:952.
8. Mehra D, Galor A. Digital Screen Use and Dry Eye: A Review. Asia Pac J Ophthalmol (Phila). 2020 Dec;9:491-497.
9. Yazdani M, Elgstøen KBP, Utheim TP. Eye Make-up Products and Dry Eye Disease: A Mini Review. Curr Eye Res. 2022 Jan;47:1-11.
10. Efron N, Brennan NA, Bright FV, et al. Contact lens care and ocular surface homeostasis. Cont Lens Anterior Eye. 2013 Jan 15;36 Suppl 1:S9-S13.
11. Ifrah R, Quevedo L, Gantz L. Topical review of the relationship between contact lens wear and meibomian gland dysfunction. J Optom. 2023 Jan-Mar;16:12-19.
12. Stapleton F, Bakkar M, Carnt N, et al. CLEAR - Contact lens complications. Cont Lens Anterior Eye. 2021 Apr;44:330-367.
13. Carracedo G, Martin-Gil A, Fonseca B, Pintor J. Effect of overnight orthokeratology on conjunctival goblet cells. Cont Lens Anterior Eye. 2016 Aug;39:266-269.
14. García-Porta N, Rico-Del-Viejo L, Martin-Gil A, Carracedo G, Pintor J, González-Méijome JM. Differences in Dry Eye Questionnaire Symptoms in Two Different Modalities of Contact Lens Wear: Silicone-Hydrogel in Daily Wear Basis and Overnight Orthokeratology. Biomed Res Int. 2016;1242845.
15. Bavinger JC, DeLoss K, Mian SI. Scleral lens use in dry eye syndrome. Curr Opin Ophthalmol. 2015 Jul;26:319-324.
16. Harbiyeli II, Bozkurt B, Erdem E, et al. Associations with meibomian gland loss in soft and rigid contact lens wearers. Cont Lens Anterior Eye. 2022 Feb;45:101400.
17. Yang L, Zhang L, Jian Hu R, Yu PP, Jin X. The influence of overnight orthokeratology on ocular surface and dry eye-related cytokines IL-17A, IL-6, and PGE2 in children. Cont Lens Anterior Eye. 2021 Feb;44:81-88.
18. Miao S, Yan R, Jia Y, Pan Z. Effect of Intense Pulsed Light Therapy in Dry Eye Disease Caused by Meibomian Gland Dysfunction: A Systematic Review and Meta-Analysis. Eye Contact Lens. 2022 Oct 1;48:424-429.
19. Sambursky R, Davitt WF 3rd, Latkany R, et al. Sensitivity and specificity of a point-of-care matrix metalloproteinase 9 immunoassay for diagnosing inflammation related to dry eye. JAMA Ophthalmol. 2013 Jan;131:24-28.
20. de Paiva CS, Pflugfelder SC, Ng SM, Akpek EK. Topical cyclosporine A therapy for dry eye syndrome. Cochrane Database Syst Rev. 2019 Sep 13;9:CD010051.
21. Li JX, Tsai YY, Lai CT, Li YL, Wu YH, Chiang CC. Lifitegrast Ophthalmic Solution 5% Is a Safe and Efficient Eyedrop for Dry Eye Disease: A Systematic Review and Meta-Analysis. J Clin Med. 2022 Aug 26;11:5014.
22. Wong J, Lan W, Ong LM, Tong L. Non-hormonal systemic medications and dry eye. Ocul Surf. 2011 Oct;9:212-226.
23. Nijm LM, Schweitzer J, Blackmore JG. Glaucoma and Dry Eye Disease: Opportunity to Assess and Treat. Clin Ophthalmol. 2023 Oct 17;17:3063-3076.
24. Yu K, Bunya V, Maguire M, Asbell P, Ying GS; Dry Eye Assessment and Management Study Research Group. Systemic Conditions Associated with Severity of Dry Eye Signs and Symptoms in the Dry Eye Assessment and Management Study. Ophthalmology. 2021 Oct;128:1384-1392.
25. Beckman KA, Luchs J, Milner MS. Making the diagnosis of Sjögren’s syndrome in patients with dry eye. Clin Ophthalmol. 2015 Dec 24;10:43-53.
26. Gaddie IB, Donnenfeld ED, Karpecki P, et al. Saturn-2 Study Group. Lotilaner Ophthalmic Solution 0.25% for Demodex Blepharitis: Randomized, Vehicle-Controlled, Multicenter, Phase 3 Trial (Saturn-2). Ophthalmology. 2023 Oct;130:1015-1023.
27. Mohammad-Rabei H, Arabi A, Shahraki T, Rezaee-Alam Z, Baradaran-Rafii A. Role of Blepharoexfoliation in Demodex Blepharitis: A Randomized Comparative Study. Cornea. 2023 Jan 1;42:44-51.
28. Jabbour S, Ashton C, Balal S, Kaye A, Ahmad S. The management of neurotrophic keratitis. Curr Opin Ophthalmol. 2021 Jul 1;32:362-368.
29. Goyal S, Hamrah P. Understanding Neuropathic Corneal Pain--Gaps and Current Therapeutic Approaches. Semin Ophthalmol. 2016;31:59-70.
30. Asiedu K. Neurophysiology of corneal neuropathic pain and emerging pharmacotherapeutics. J Neurosci Res. 2024 Jan;102:e25285.